Central dysmyelination reduces the temporal fidelity of synaptic transmission and the reliability of postsynaptic firing during high-frequency stimulation
- PMID: 23843435
- PMCID: PMC4042425
- DOI: 10.1152/jn.00117.2013
Central dysmyelination reduces the temporal fidelity of synaptic transmission and the reliability of postsynaptic firing during high-frequency stimulation
Abstract
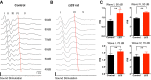
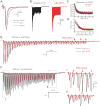
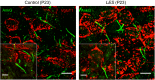
Auditory brain stem circuits rely on fast, precise, and reliable neurotransmission to process auditory information. To determine the fundamental role of myelination in auditory brain stem function, we examined the evoked auditory brain stem response (ABR) from the Long Evans shaker (LES) rat, which lacks myelin due to a genetic deletion of myelin basic protein. In control rats, the ABR evoked by a click consisted of five well-defined waves (denoted waves I-V). In LES rats, waves I, IV, and V were present, but waves II and III were undetectable, indicating disrupted function in the earliest stages of central nervous system auditory processing. In addition, the developmental shortening of the interval between waves I and IV that normally occurs in control rats was arrested and resulted in a significant increase in the central conduction time in LES rats. In brain stem slices, action potential transmission between the calyx of Held terminals and the medial nucleus of the trapezoid body (MNTB) neurons was delayed and less reliable in LES rats, although the resting potential, threshold, input resistance, and length of the axon initial segment of the postsynaptic MNTB neurons were normal. The amplitude of glutamatergic excitatory postsynaptic currents (EPSCs) and the degree of synaptic depression during high-frequency stimulation were not different between LES rats and controls, but LES rats exhibited a marked slow component to the EPSC decay and a much higher rate of presynaptic failures. Together, these results indicate that loss of myelin disrupts brain stem auditory processing, increasing central conduction time and reducing the reliability of neurotransmission.
Keywords: ABR; MNTB principal neuron; auditory brain stem; auditory neuropathy; calyx of Held synapse; myelin.
Figures




Similar articles
-
SK Channels Regulate Resting Properties and Signaling Reliability of a Developing Fast-Spiking Neuron.J Neurosci. 2017 Nov 1;37(44):10738-10747. doi: 10.1523/JNEUROSCI.1243-17.2017. Epub 2017 Oct 5. J Neurosci. 2017. PMID: 28982705 Free PMC article.
-
Developmental profiles of the intrinsic properties and synaptic function of auditory neurons in preterm and term baboon neonates.J Neurosci. 2014 Aug 20;34(34):11399-404. doi: 10.1523/JNEUROSCI.4734-13.2014. J Neurosci. 2014. PMID: 25143619 Free PMC article.
-
Non-calyceal excitatory inputs mediate low fidelity synaptic transmission in rat auditory brainstem slices.Eur J Neurosci. 2003 Nov;18(10):2899-902. doi: 10.1111/j.1460-9568.2003.03017.x. Eur J Neurosci. 2003. PMID: 14656340
-
The ion channels and synapses responsible for the physiological diversity of mammalian lower brainstem auditory neurons.Hear Res. 2019 May;376:33-46. doi: 10.1016/j.heares.2018.12.011. Epub 2018 Dec 26. Hear Res. 2019. PMID: 30606624 Review.
-
The calyx of Held synapse: from model synapse to auditory relay.Annu Rev Physiol. 2012;74:199-224. doi: 10.1146/annurev-physiol-020911-153236. Epub 2011 Oct 24. Annu Rev Physiol. 2012. PMID: 22035348 Review.
Cited by
-
Impact of Auditory Experience on the Structural Plasticity of the AIS in the Mouse Brainstem Throughout the Lifespan.Front Cell Neurosci. 2019 Oct 15;13:456. doi: 10.3389/fncel.2019.00456. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31680869 Free PMC article.
-
Loss of β4-spectrin impairs Nav channel clustering at the heminode and temporal fidelity of presynaptic spikes in developing auditory brain.Sci Rep. 2022 Apr 7;12(1):5854. doi: 10.1038/s41598-022-09856-9. Sci Rep. 2022. PMID: 35393465 Free PMC article.
-
A new mouse model of Canavan leukodystrophy displays hearing impairment due to central nervous system dysmyelination.Dis Model Mech. 2014 Jun;7(6):649-57. doi: 10.1242/dmm.014605. Epub 2014 Mar 28. Dis Model Mech. 2014. PMID: 24682784 Free PMC article.
-
Myelination of Purkinje axons is critical for resilient synaptic transmission in the deep cerebellar nucleus.Sci Rep. 2018 Jan 18;8(1):1022. doi: 10.1038/s41598-018-19314-0. Sci Rep. 2018. PMID: 29348594 Free PMC article.
-
Axon-glia interactions in the ascending auditory system.Dev Neurobiol. 2021 Jul;81(5):546-567. doi: 10.1002/dneu.22813. Epub 2021 Feb 26. Dev Neurobiol. 2021. PMID: 33561889 Free PMC article. Review.
References
-
- Bennett V, Lambert S. Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol 28: 303–318, 1999 - PubMed
-
- Carr CE, Soares D, Parameshwaran S, Perney T. Evolution and development of time coding systems. Curr Opin Neurobiol 11: 727–733, 2001 - PubMed
-
- Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, Musella A, D'Amelio M, Cavallucci V, Martorana A, Bergamaschi A, Cencioni MT, Diamantini A, Butti E, Comi G, Bernardi G, Cecconi F, Battistini L, Furlan R, Martino G. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci 29: 3442–3452, 2009 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

