Dopaminergic modulation of GABAergic transmission in the entorhinal cortex: concerted roles of α1 adrenoreceptors, inward rectifier K⁺, and T-type Ca²⁺ channels
- PMID: 23843440
- PMCID: PMC4296123
- DOI: 10.1093/cercor/bht177
Dopaminergic modulation of GABAergic transmission in the entorhinal cortex: concerted roles of α1 adrenoreceptors, inward rectifier K⁺, and T-type Ca²⁺ channels
Abstract
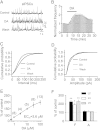
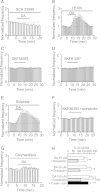
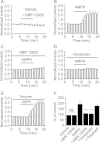
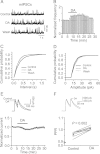
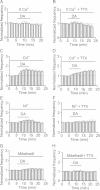
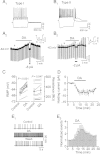
Whereas the entorhinal cortex (EC) receives profuse dopaminergic innervations from the midbrain, the effects of dopamine (DA) on γ-Aminobutyric acid (GABA)ergic interneurons in this brain region have not been determined. We probed the actions of DA on GABAA receptor-mediated synaptic transmission in the EC. Application of DA increased the frequency, not the amplitude, of spontaneous IPSCs (sIPSCs) and miniature IPSCs (mIPSCs) recorded from entorhinal principal neurons, but slightly reduced the amplitude of the evoked IPSCs. The effects of DA were unexpectedly found to be mediated by α1 adrenoreceptors, but not by DA receptors. DA endogenously released by the application of amphetamine also increased the frequency of sIPSCs. Ca(2+) influx via T-type Ca(2+) channels was required for DA-induced facilitation of sIPSCs and mIPSCs. DA depolarized and enhanced the firing frequency of action potentials of interneurons. DA-induced depolarization was independent of extracellular Na(+) and Ca(2+) and did not require the functions of hyperpolarization-activated (Ih) channels and T-type Ca(2+) channels. DA-generated currents showed a reversal potential close to the K(+) reversal potential and inward rectification, suggesting that DA inhibits the inward rectifier K(+) channels (Kirs). Our results demonstrate that DA facilitates GABA release by activating α1 adrenoreceptors to inhibit Kirs, which further depolarize interneurons resulting in secondary Ca(2+) influx via T-type Ca(+) channels.
Keywords: GABAA receptor; depolarization; entorhinal; interneuron; synapse; synaptic transmission.
© The Author 2013. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Akil M, Lewis DA. The dopaminergic innervation of monkey entorhinal cortex. Cereb Cortex. 1993;3:533–550. - PubMed
-
- Anfossi G, Massucco P, Mularoni E, Mattiello L, Cavalot F, Burzacca S, Trovati M. Effect of dopamine on adenosine 3′,5′-cyclic monophosphate levels in human platelets. Gen Pharmacol. 1993;24:435–438. - PubMed
-
- Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–632. - PubMed
-
- Avoli M, D'Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

