Heparin dodecasaccharide containing two antithrombin-binding pentasaccharides: structural features and biological properties
- PMID: 23843463
- PMCID: PMC3764794
- DOI: 10.1074/jbc.M113.485268
Heparin dodecasaccharide containing two antithrombin-binding pentasaccharides: structural features and biological properties
Abstract
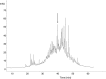
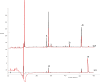
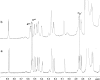
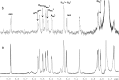
The antithrombin (AT) binding properties of heparin and low molecular weight heparins are strongly associated to the presence of the pentasaccharide sequence AGA*IA (A(NAc,6S)-GlcUA-A(NS,3,6S)-I(2S)-A(NS,6S)). By using the highly chemoselective depolymerization to prepare new ultra low molecular weight heparin and coupling it with the original separation techniques, it was possible to isolate a polysaccharide with a biosynthetically unexpected structure and excellent antithrombotic properties. It consisted of a dodecasaccharide containing an unsaturated uronate unit at the nonreducing end and two contiguous AT-binding sequences separated by a nonsulfated iduronate residue. This novel oligosaccharide was characterized by NMR spectroscopy, and its binding with AT was determined by fluorescence titration, NMR, and LC-MS. The dodecasaccharide displayed a significantly increased anti-FXa activity compared with those of the pentasaccharide, fondaparinux, and low molecular weight heparin enoxaparin.
Keywords: 3-O-Sulfation; Antithrombin; Glycosaminoglycan; Heparin; Mass Spectrometry (MS); NMR.
Figures



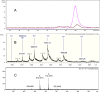
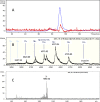
References
-
- Barowcliffe T. W. (2012) in Heparin: A Century of Progress (Lever R., Mulloy B., Page C. P., eds) pp. 3–22, Springer-Verlag, Berlin
-
- Lever R., Page C. P. (2012) Heparin: A Century of Progress (Lever R., Mulloy B., Page C. P., eds) pp. 281–306, Springer-Verlag, Berlin
-
- Thunberg L., Bäckström G., Lindahl U. (1982) Further characterization of the antithrombin-binding sequence in heparin. Carbohydr. Res. 100, 393–410 - PubMed
-
- Jeske W., Lormeau J. C., Callas D., Iqbal O., Hoppensteadt D., Fareed J. (1995) Antithrombin III affinity dependence on the anticoagulant, antiprotease, and tissue factor pathway inhibitor actions of heparins. Semin. Thromb. Hemost. 21, 193–200 - PubMed
-
- Petitou M., van Boeckel C. A. (2004) A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew. Chem. Int. Ed. Engl. 43, 3118–3133 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

