A sensitive multiplex, real-time PCR assay for prospective detection of Shiga toxin-producing Escherichia coli from stool samples reveals similar incidences but variable severities of non-O157 and O157 infections in northern California
- PMID: 23843484
- PMCID: PMC3754673
- DOI: 10.1128/JCM.00991-13
A sensitive multiplex, real-time PCR assay for prospective detection of Shiga toxin-producing Escherichia coli from stool samples reveals similar incidences but variable severities of non-O157 and O157 infections in northern California
Abstract
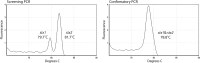
Rapid and accurate detection of Shiga toxin-producing Escherichia coli (STEC) of all serotypes from patients with diarrhea is critical for medical management and for the prevention of ongoing transmission. In this prospective study, we assessed the performance of a multiplex, real-time PCR assay targeting stx1 and stx2 for the detection of O157 and non-O157 STEC in diarrheal stool samples enriched in Gram-negative broth. We show that the assay is 100% sensitive (95% confidence interval [CI], 89.1% to 100%) and 98.5% specific (95% CI, 90.6% to 99.9%) based on a panel of 40 known STEC-positive specimens and 65 known negative specimens. During a 2-year postvalidation period, the assay detected more positive samples from patients in northern California than did culture and PCR testing performed at a public health reference laboratory, with a positive predictive value of 95.6% (95% CI, 87.6% to 99.1%). Serotyping data showed an incidence rate of 51.2% for non-O157 STEC strains, with 5.8% of patients (1/17) with non-O157 strains and 42.9% (6/14) with O157 strains (P = 0.03) developing hemolytic-uremic syndrome. The findings from this study underscore the recommendations of the CDC for laboratories to test all diarrheal stool samples from patients with acute community-acquired diarrhea for non-O157 STEC in addition to the O157 serotype by using a sensitive assay. Additionally, a survey of 17 clinical laboratories in northern California demonstrated that nearly 50% did not screen all stool specimens for the presence of Shiga toxins, indicating that many clinical microbiology laboratories still do not routinely screen all stool specimens for the presence of Shiga toxins as recommended in the 2009 CDC guidelines.
Figures
Similar articles
-
Real-Time PCR Assay for Detection and Differentiation of Shiga Toxin-Producing Escherichia coli from Clinical Samples.J Clin Microbiol. 2015 Jul;53(7):2148-53. doi: 10.1128/JCM.00115-15. Epub 2015 Apr 29. J Clin Microbiol. 2015. PMID: 25926491 Free PMC article.
-
Characteristics of O157 versus non-O157 Shiga toxin-producing Escherichia coli infections in Minnesota, 2000-2006.Clin Infect Dis. 2009 Aug 1;49(3):358-64. doi: 10.1086/600302. Clin Infect Dis. 2009. PMID: 19548834
-
Evaluation of performance and potential clinical impact of ProSpecT Shiga toxin Escherichia coli microplate assay for detection of Shiga Toxin-producing E. coli in stool samples.J Clin Microbiol. 2004 Apr;42(4):1652-6. doi: 10.1128/JCM.42.4.1652-1656.2004. J Clin Microbiol. 2004. PMID: 15071021 Free PMC article.
-
Non-O157 Shiga toxin-producing Escherichia coli in foods.J Food Prot. 2010 Sep;73(9):1721-36. doi: 10.4315/0362-028x-73.9.1721. J Food Prot. 2010. PMID: 20828483 Review.
-
Review of the role of gastrointestinal multiplex polymerase chain reaction in the management of diarrheal illness.J Gastroenterol Hepatol. 2021 Dec;36(12):3286-3297. doi: 10.1111/jgh.15581. Epub 2021 Jun 23. J Gastroenterol Hepatol. 2021. PMID: 34129249 Review.
Cited by
-
Shiga Toxin-Producing Escherichia coli in Diarrheal Stool of Swedish Children: Evaluation of Polymerase Chain Reaction Screening and Duration of Shiga Toxin Shedding.J Pediatric Infect Dis Soc. 2016 Jun;5(2):147-51. doi: 10.1093/jpids/piv003. Epub 2015 Feb 17. J Pediatric Infect Dis Soc. 2016. PMID: 27199470 Free PMC article.
-
Real-Time PCR Assay for Detection and Differentiation of Shiga Toxin-Producing Escherichia coli from Clinical Samples.J Clin Microbiol. 2015 Jul;53(7):2148-53. doi: 10.1128/JCM.00115-15. Epub 2015 Apr 29. J Clin Microbiol. 2015. PMID: 25926491 Free PMC article.
-
Pooled Nucleic Acid Amplification Test for Screening of Stool Specimens for Shiga Toxin-Producing Escherichia coli.J Clin Microbiol. 2016 Nov;54(11):2711-2715. doi: 10.1128/JCM.01373-16. Epub 2016 Aug 24. J Clin Microbiol. 2016. PMID: 27558177 Free PMC article.
-
Colorimetric dual DNAzyme reaction triggered by loop-mediated isothermal amplification for the visual detection of Shiga toxin-producing Escherichia coli in food matrices.PLoS One. 2025 Apr 23;20(4):e0320393. doi: 10.1371/journal.pone.0320393. eCollection 2025. PLoS One. 2025. PMID: 40267081 Free PMC article.
-
Multiplex real-time PCR assay for detection of Escherichia coli O157:H7 and screening for non-O157 Shiga toxin-producing E. coli.BMC Microbiol. 2017 Nov 9;17(1):215. doi: 10.1186/s12866-017-1123-2. BMC Microbiol. 2017. PMID: 29121863 Free PMC article.
References
-
- Gyles CL. 2007. Shiga toxin-producing Escherichia coli: an overview. J. Anim. Sci. 85:E45–E62 - PubMed
-
- Hadler JL, Clogher P, Hurd S, Phan Q, Mandour M, Bemis K, Marcus R. 2011. Ten-year trends and risk factors for non-O157 Shiga toxin-producing Escherichia coli found through Shiga toxin testing, Connecticut, 2000–2009. Clin. Infect. Dis. 53:269–276 - PubMed
-
- Hedican EB, Medus C, Besser JM, Juni BA, Koziol B, Taylor C, Smith KE. 2009. Characteristics of O157 versus non-O157 Shiga toxin-producing Escherichia coli infections in Minnesota, 2000–2006. Clin. Infect. Dis. 49:358–364 - PubMed
-
- Bielaszewska M, Mellmann A, Zhang W, Kock R, Fruth A, Bauwens A, Peters G, Karch H. 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect. Dis. 11:671–676 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

