Layer 4 in primary visual cortex of the awake rabbit: contrasting properties of simple cells and putative feedforward inhibitory interneurons
- PMID: 23843510
- PMCID: PMC3724558
- DOI: 10.1523/JNEUROSCI.0863-13.2013
Layer 4 in primary visual cortex of the awake rabbit: contrasting properties of simple cells and putative feedforward inhibitory interneurons
Abstract
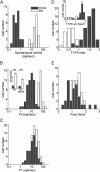
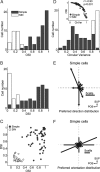
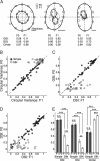
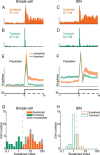
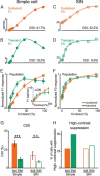
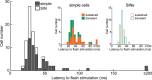
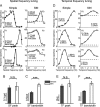
Extracellular recordings were obtained from two cell classes in layer 4 of the awake rabbit primary visual cortex (V1): putative inhibitory interneurons [suspected inhibitory interneurons (SINs)] and putative excitatory cells with simple receptive fields. SINs were identified solely by their characteristic response to electrical stimulation of the lateral geniculate nucleus (LGN, 3+ spikes at >600 Hz), and simple cells were identified solely by receptive field structure, requiring spatially separate ON and/or OFF subfields. Notably, no cells met both criteria, and we studied 62 simple cells and 33 SINs. Fourteen cells met neither criterion. These layer 4 populations were markedly distinct. Thus, SINs were far less linear (F1/F0 < 1), more broadly tuned to stimulus orientation, direction, spatial and temporal frequency, more sensitive to contrast, had much higher spontaneous and stimulus-driven activity, and always had spatially overlapping ON/OFF receptive subfields. SINs responded to drifting gratings with increased firing rates (F0) for all orientations and directions. However, some SINs showed a weaker modulated (F1) response sharply tuned to orientation and/or direction. SINs responded at shorter latencies than simple cells to stationary stimuli, and the responses of both populations could be sustained or transient. Transient simple cells were more sensitive to contrast than sustained simple cells and their visual responses were more frequently suppressed by high contrasts. Finally, cross-correlation between LGN and SIN spike trains confirmed a fast and precisely timed monosynaptic connectivity, supporting the notion that SINs are well suited to provide a fast feedforward inhibition onto targeted cortical populations.
Figures





Similar articles
-
Fast-Spike Interneurons in Visual Cortical Layer 5: Heterogeneous Response Properties Are Related to Thalamocortical Connectivity.J Neurosci. 2025 Jan 22;45(4):e1116242024. doi: 10.1523/JNEUROSCI.1116-24.2024. J Neurosci. 2025. PMID: 39667901 Free PMC article.
-
Corticogeniculate neurons, corticotectal neurons, and suspected interneurons in visual cortex of awake rabbits: receptive-field properties, axonal properties, and effects of EEG arousal.J Neurophysiol. 1987 Apr;57(4):977-1001. doi: 10.1152/jn.1987.57.4.977. J Neurophysiol. 1987. PMID: 3585466
-
Fast-spike interneurons and feedforward inhibition in awake sensory neocortex.Cereb Cortex. 2003 Jan;13(1):25-32. doi: 10.1093/cercor/13.1.25. Cereb Cortex. 2003. PMID: 12466212 Review.
-
Influence of VPM afferents on putative inhibitory interneurons in S1 of the awake rabbit: evidence from cross-correlation, microstimulation, and latencies to peripheral sensory stimulation.J Neurophysiol. 1995 Apr;73(4):1584-99. doi: 10.1152/jn.1995.73.4.1584. J Neurophysiol. 1995. PMID: 7643169
-
Thalamocortical control of feed-forward inhibition in awake somatosensory 'barrel' cortex.Philos Trans R Soc Lond B Biol Sci. 2002 Dec 29;357(1428):1717-27. doi: 10.1098/rstb.2002.1156. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12626006 Free PMC article. Review.
Cited by
-
Testing the odds of inherent vs. observed overdispersion in neural spike counts.J Neurophysiol. 2016 Jan 1;115(1):434-44. doi: 10.1152/jn.00194.2015. Epub 2015 Oct 7. J Neurophysiol. 2016. PMID: 26445864 Free PMC article.
-
GABAergic synapses: their plasticity and role in sensory cortex.Front Cell Neurosci. 2014 Mar 26;8:91. doi: 10.3389/fncel.2014.00091. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24723851 Free PMC article. Review.
-
Visual Corticotectal Neurons in Awake Rabbits: Receptive Fields and Driving Monosynaptic Thalamocortical Inputs.J Neurosci. 2024 May 8;44(19):e1945232024. doi: 10.1523/JNEUROSCI.1945-23.2024. J Neurosci. 2024. PMID: 38485258 Free PMC article.
-
Functional implications of orientation maps in primary visual cortex.Nat Commun. 2016 Nov 23;7:13529. doi: 10.1038/ncomms13529. Nat Commun. 2016. PMID: 27876796 Free PMC article.
-
Axonal Conduction Delays, Brain State, and Corticogeniculate Communication.J Neurosci. 2017 Jun 28;37(26):6342-6358. doi: 10.1523/JNEUROSCI.0444-17.2017. Epub 2017 May 30. J Neurosci. 2017. PMID: 28559382 Free PMC article.
References
-
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
