Reevaluation of the beam and radial hypotheses of parallel fiber action in the cerebellar cortex
- PMID: 23843513
- PMCID: PMC3724546
- DOI: 10.1523/JNEUROSCI.0711-13.2013
Reevaluation of the beam and radial hypotheses of parallel fiber action in the cerebellar cortex
Abstract
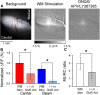
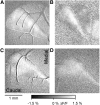
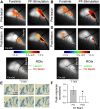
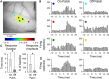
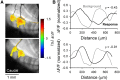
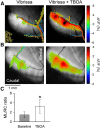
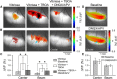
The role of parallel fibers (PFs) in cerebellar physiology remains controversial. Early studies inspired the "beam" hypothesis whereby granule cell (GC) activation results in PF-driven, postsynaptic excitation of beams of Purkinje cells (PCs). However, the "radial" hypothesis postulates that the ascending limb of the GC axon provides the dominant input to PCs and generates patch-like responses. Using optical imaging and single-cell recordings in the mouse cerebellar cortex in vivo, this study reexamines the beam versus radial controversy. Electrical stimulation of mossy fibers (MFs) as well as microinjection of NMDA in the granular layer generates beam-like responses with a centrally located patch-like response. Remarkably, ipsilateral forepaw stimulation evokes a beam-like response in Crus I. Discrete molecular layer lesions demonstrate that PFs contribute to the peripherally generated responses in Crus I. In contrast, vibrissal stimulation induces patch-like activation of Crus II and GABAA antagonists fail to convert this patch-like activity into a beam-like response, implying that molecular layer inhibition does not prevent beam-like responses. However, blocking excitatory amino acid transporters (EAATs) generates beam-like responses in Crus II. These beam-like responses are suppressed by focal inhibition of MF-GC synaptic transmission. Using EAAT4 reporter transgenic mice, we show that peripherally evoked patch-like responses in Crus II are aligned between parasagittal bands of EAAT4. This is the first study to demonstrate beam-like responses in the cerebellar cortex to peripheral, MF, and GC stimulation in vivo. Furthermore, the spatial pattern of the responses depends on extracellular glutamate and its local regulation by EAATs.
Figures



Similar articles
-
Spatial distribution of field potential profiles in the cat cerebellar cortex evoked by peripheral and central inputs.Neuroscience. 1997 Dec;81(4):1155-81. doi: 10.1016/s0306-4522(97)00255-8. Neuroscience. 1997. PMID: 9330375
-
Imaging parallel fiber and climbing fiber responses and their short-term interactions in the mouse cerebellar cortex in vivo.Neuroscience. 2004;126(1):213-27. doi: 10.1016/j.neuroscience.2004.02.020. Neuroscience. 2004. PMID: 15145087
-
Optical imaging of parallel fiber activation in the rat cerebellar cortex: spatial effects of excitatory amino acids.Neuroscience. 1993 Feb;52(4):771-86. doi: 10.1016/0306-4522(93)90528-n. Neuroscience. 1993. PMID: 8095709
-
Excitation and Inhibition Delays within a Feedforward Inhibitory Pathway Modulate Cerebellar Purkinje Cell Output in Mice.J Neurosci. 2023 Aug 16;43(33):5905-5917. doi: 10.1523/JNEUROSCI.0091-23.2023. Epub 2023 Jul 26. J Neurosci. 2023. PMID: 37495382 Free PMC article.
-
Glutamate-receptor-like molecule GluRδ2 involved in synapse formation at parallel fiber-Purkinje neuron synapses.Cerebellum. 2012 Mar;11(1):71-7. doi: 10.1007/s12311-010-0170-0. Cerebellum. 2012. PMID: 20387025 Review.
Cited by
-
Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits.Nat Rev Neurosci. 2015 Feb;16(2):79-93. doi: 10.1038/nrn3886. Nat Rev Neurosci. 2015. PMID: 25601779 Free PMC article. Review.
-
Neuronal activity patterns in microcircuits of the cerebellar cortical C3 zone during reaching.J Physiol. 2022 Dec;600(23):5077-5099. doi: 10.1113/JP282928. Epub 2022 Nov 9. J Physiol. 2022. PMID: 36254104 Free PMC article.
-
An excitatory GABA loop operating in vivo.Front Cell Neurosci. 2015 Jul 16;9:275. doi: 10.3389/fncel.2015.00275. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26236197 Free PMC article.
-
Primate cerebellar granule cells exhibit a tonic GABAAR conductance that is not affected by alcohol: a possible cellular substrate of the low level of response phenotype.Front Neural Circuits. 2013 Nov 26;7:189. doi: 10.3389/fncir.2013.00189. eCollection 2013. Front Neural Circuits. 2013. PMID: 24324408 Free PMC article.
-
Purkinje Cell Spatial Correlation Dynamics Are Key to Cerebellar Cortical Contributions to Behavior.J Neurosci. 2025 Jul 23;45(30):e1915242025. doi: 10.1523/JNEUROSCI.1915-24.2025. J Neurosci. 2025. PMID: 40555517
References
-
- Bell CC, Grimm RJ. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969;32:1044–1055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
