Rapid suppression of inhibitory synaptic transmission by retinoic acid
- PMID: 23843516
- PMCID: PMC3724332
- DOI: 10.1523/JNEUROSCI.1710-13.2013
Rapid suppression of inhibitory synaptic transmission by retinoic acid
Abstract
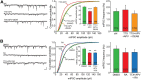
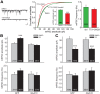
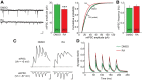
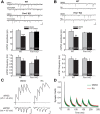
In brain, properly balanced synaptic excitation and inhibition is critically important for network stability and efficient information processing. Here, we show that retinoic acid (RA), a synaptic signaling molecule whose synthesis is activated by reduced neural activity, induces rapid internalization of synaptic GABAA receptors in mouse hippocampal neurons, leading to significant reduction of inhibitory synaptic transmission. Similar to its action at excitatory synapses, action of RA at inhibitory synapses requires protein translation and is mediated by a nontranscriptional function of the RA-receptor RARα. Different from RA action at excitatory synapses, however, RA at inhibitory synapses causes a loss instead of the gain of a synaptic protein (i.e., GABAARs). Moreover, the removal of GABAARs from the synapses and the reduction of synaptic inhibition do not require the execution of RA's action at excitatory synapses (i.e., downscaling of synaptic inhibition is intact when upscaling of synaptic excitation is blocked). Thus, the action of RA at inhibitory and excitatory synapses diverges significantly after the step of RARα-mediated protein synthesis, and the regulations of GABAAR and AMPAR trafficking are independent processes. When both excitatory and inhibitory synapses are examined together in the same neuron, the synaptic excitation/inhibition ratio is significantly enhanced by RA. Importantly, RA-mediated downscaling of synaptic inhibition is completely absent in Fmr1 knock-out neurons. Thus, RA acts as a central organizer for coordinated homeostatic plasticity in both excitatory and inhibitory synapses, and impairment of this overall process alters the excitatory/inhibitory balance of a circuit and likely represents a major feature of fragile X-syndrome.
Figures



References
-
- Brandon NJ, Jovanovic JN, Colledge M, Kittler JT, Brandon JM, Scott JD, Moss SJ. A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABA(A) receptors by cAMP-dependent protein kinase via selective interaction with receptor beta subunits. Mol Cell Neurosci. 2003;22:87–97. doi: 10.1016/S1044-7431(02)00017-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
