Evidence for a gender-specific protective role of innate immune receptors in a model of perinatal brain injury
- PMID: 23843525
- PMCID: PMC6618687
- DOI: 10.1523/JNEUROSCI.0535-13.2013
Evidence for a gender-specific protective role of innate immune receptors in a model of perinatal brain injury
Abstract
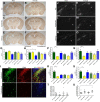
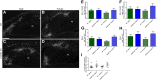
Hypoxia-ischemia is a common cause of neurological impairments in newborns, but little is known about how neuroinflammation contributes to the long-term outcome after a perinatal brain injury. In this study, we investigated the role of the fractalkine receptor chemokine CX3C motif receptor 1 (CX3CR1) and of toll-like receptor (TLR) signaling after a neonatal hypoxic-ischemic brain injury. Mice deficient in the TLR adaptor proteins Toll/interleukin-1 receptor-domain-containing adaptor protein inducing interferon β (TRIF) or myeloid differentiation factor-88 (MyD88) and CX3CR1 knock-out (KO) mice were subjected to hypoxia-ischemia at postnatal day 3. In situ hybridization was used to evaluate the expression of TLRs during brain development and after hypoxic-ischemic insults. Behavioral deficits, hippocampal damage, reactive microgliosis, and subplate injury were compared among the groups. Although MyD88 KO mice exhibited no differences from wild-type animals in long-term structural and functional outcomes, TRIF KO mice presented a worse outcome, as evidenced by increased hippocampal CA3 atrophy in males and by the development of learning and motor deficits in females. CX3CR1-deficient female mice showed a marked increase in brain damage and long-lasting learning deficits, whereas CX3CR1 KO male animals did not exhibit more brain injury than wild-type mice. These data reveal a novel, gender-specific protective role of TRIF and CX3CR1 signaling in a mouse model of neonatal hypoxic-ischemic brain injury. These findings suggest that future studies seeking immunomodulatory therapies for preterm infants should consider gender as a critical variable and should be cautious not to abrogate the protective role of neuroinflammation.
Figures


References
-
- Beaino G, Khoshnood B, Kaminski M, Pierrat V, Marret S, Matis J, Ledésert B, Thiriez G, Fresson J, Roz é JC, Zupan-Simunek V, Arnaud C, Burguet A, Larroque B, Bréart G, Ancel PY. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol. 2010;52:e119–125. doi: 10.1111/j.1469-8749.2010.03612.x. - DOI - PubMed
-
- Cameron JS, Alexopoulou L, Sloane JA, DiBernardo AB, Ma Y, Kosaras B, Flavell R, Strittmatter SM, Volpe J, Sidman R, Vartanian T. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J Neurosci. 2007;27:13033–13041. doi: 10.1523/JNEUROSCI.4290-06.2007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
