Tocopherol derivative TFA-12 promotes myelin repair in experimental models of multiple sclerosis
- PMID: 23843531
- PMCID: PMC6618682
- DOI: 10.1523/JNEUROSCI.0774-13.2013
Tocopherol derivative TFA-12 promotes myelin repair in experimental models of multiple sclerosis
Abstract
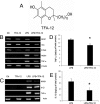
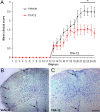
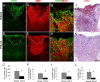
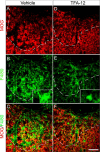
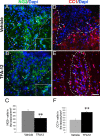
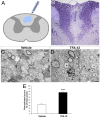
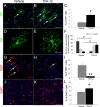
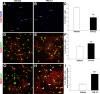
Multiple sclerosis (MS) is an inflammatory disease of the CNS that is associated with demyelination and axonal loss, resulting in severe neurological handicap. Current MS therapies mostly target neuroinflammation but have only a little impact on CNS myelin repair. Progress toward treatments that enhance remyelination would therefore represent major advances in MS treatment. Here, we examined the ability of TFA-12, a new synthetic compound belonging to tocopherol long-chain fatty alcohols, to promote oligodendrocyte regeneration and remyelination in experimental models of MS. We showed that TFA-12 significantly ameliorates neurological deficit and severity of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (EAE) in mice. Histological evaluation of mouse EAE spinal cords showed that TFA-12 treatment reduces inflammation, astrogliosis, and myelin loss. Additionally, we demonstrated that TFA-12 accelerates remyelination of focal demyelinated lesions induced by lysolecithin injections. We also found that this compound induces the differentiation of oligodendrocyte precursor cells into mature oligodendrocytes through the inhibition of the Notch/Jagged1 signaling pathway. Altogether, our data provide important proof of principle indicating that TFA-12 could be a potential therapeutic compound for myelin repair in MS.
Figures
References
-
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
