Input-output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro
- PMID: 23843535
- PMCID: PMC3724544
- DOI: 10.1523/JNEUROSCI.5729-12.2013
Input-output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro
Abstract
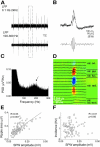
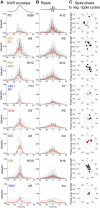
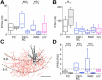
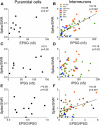
Hippocampal sharp waves and the associated ripple oscillations (SWRs) are implicated in memory processes. These network events emerge intrinsically in the CA3 network. To understand cellular interactions that generate SWRs, we detected first spiking activity followed by recording of synaptic currents in distinct types of anatomically identified CA3 neurons during SWRs that occurred spontaneously in mouse hippocampal slices. We observed that the vast majority of interneurons fired during SWRs, whereas only a small portion of pyramidal cells was found to spike. There were substantial differences in the firing behavior among interneuron groups; parvalbumin-expressing basket cells were one of the most active GABAergic cells during SWRs, whereas ivy cells were silent. Analysis of the synaptic currents during SWRs uncovered that the dominant synaptic input to the pyramidal cell was inhibitory, whereas spiking interneurons received larger synaptic excitation than inhibition. The discharge of all interneurons was primarily determined by the magnitude and the timing of synaptic excitation. Strikingly, we observed that the temporal structure of synaptic excitation and inhibition during SWRs significantly differed between parvalbumin-containing basket cells, axoaxonic cells, and type 1 cannabinoid receptor (CB1)-expressing basket cells, which might explain their distinct recruitment to these synchronous events. Our data support the hypothesis that the active current sources restricted to the stratum pyramidale during SWRs originate from the synaptic output of parvalbumin-expressing basket cells. Thus, in addition to gamma oscillation, these GABAergic cells play a central role in SWR generation.
Figures



Similar articles
-
Mechanisms of sharp wave initiation and ripple generation.J Neurosci. 2014 Aug 20;34(34):11385-98. doi: 10.1523/JNEUROSCI.0867-14.2014. J Neurosci. 2014. PMID: 25143618 Free PMC article.
-
Properties and dynamics of inhibitory synaptic communication within the CA3 microcircuits of pyramidal cells and interneurons expressing parvalbumin or cholecystokinin.J Physiol. 2016 Jul 1;594(13):3745-74. doi: 10.1113/JP272231. Epub 2016 May 5. J Physiol. 2016. PMID: 27038232 Free PMC article.
-
Feedforward inhibition underlies the propagation of cholinergically induced gamma oscillations from hippocampal CA3 to CA1.J Neurosci. 2013 Jul 24;33(30):12337-51. doi: 10.1523/JNEUROSCI.3680-12.2013. J Neurosci. 2013. PMID: 23884940 Free PMC article.
-
Generation of physiological and pathological high frequency oscillations: the role of perisomatic inhibition in sharp-wave ripple and interictal spike generation.Curr Opin Neurobiol. 2015 Apr;31:26-32. doi: 10.1016/j.conb.2014.07.020. Epub 2014 Aug 14. Curr Opin Neurobiol. 2015. PMID: 25128735 Review.
-
Network mechanisms of gamma oscillations in the CA3 region of the hippocampus.Neural Netw. 2009 Oct;22(8):1113-9. doi: 10.1016/j.neunet.2009.07.024. Epub 2009 Jul 22. Neural Netw. 2009. PMID: 19683412 Review.
Cited by
-
Direct Medial Entorhinal Cortex Input to Hippocampal CA3 Is Crucial for eEF2K Inhibitor-Induced Neuronal Oscillations in the Mouse Hippocampus.Front Cell Neurosci. 2020 Mar 6;14:24. doi: 10.3389/fncel.2020.00024. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32210764 Free PMC article.
-
Hippocampal Ripple Oscillations and Inhibition-First Network Models: Frequency Dynamics and Response to GABA Modulators.J Neurosci. 2018 Mar 21;38(12):3124-3146. doi: 10.1523/JNEUROSCI.0188-17.2018. Epub 2018 Feb 16. J Neurosci. 2018. PMID: 29453207 Free PMC article.
-
Transient Oxygen-Glucose Deprivation Causes Region- and Cell Type-Dependent Functional Deficits in the Mouse Hippocampus In Vitro.eNeuro. 2021 Sep 29;8(5):ENEURO.0221-21.2021. doi: 10.1523/ENEURO.0221-21.2021. Print 2021 Sep-Oct. eNeuro. 2021. PMID: 34475264 Free PMC article.
-
Synaptic Reorganization of the Perisomatic Inhibitory Network in Hippocampi of Temporal Lobe Epileptic Patients.Biomed Res Int. 2017;2017:7154295. doi: 10.1155/2017/7154295. Epub 2017 Jan 2. Biomed Res Int. 2017. PMID: 28116310 Free PMC article. Review.
-
Hyperexcitability of the network contributes to synchronization processes in the human epileptic neocortex.J Physiol. 2018 Jan 15;596(2):317-342. doi: 10.1113/JP275413. Epub 2017 Dec 28. J Physiol. 2018. PMID: 29178354 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
