Response selectivity is correlated to dendritic structure in parvalbumin-expressing inhibitory neurons in visual cortex
- PMID: 23843539
- PMCID: PMC3724550
- DOI: 10.1523/JNEUROSCI.2196-12.2013
Response selectivity is correlated to dendritic structure in parvalbumin-expressing inhibitory neurons in visual cortex
Abstract
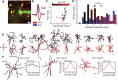
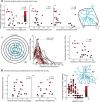
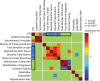
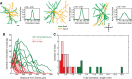
Inhibitory neurons have been shown to perform a variety of functions within brain circuits, including shaping response functions in target cells. Still, how the properties of specific inhibitory neuron classes relate to their local circuits remains unclear. To better understand the distribution and origins of orientation selectivity in inhibitory neurons expressing the calcium binding protein parvalbumin (PV) in the mouse primary visual cortex, we labeled PV(+) neurons with red fluorescent protein (RFP) and targeted them for cell-attached electrophysiological recordings. PV(+) neurons could be broadly tuned or sharply tuned for orientation but tended to be more broadly tuned than unlabeled neurons on average. The dendritic morphology of PV(+) cells, revealed by intracellular labeling, was strongly correlated with tuning: highly tuned PV(+) neurons had shorter dendrites that branched nearer to the soma and had smaller dendritic fields overall, whereas broadly tuned PV(+) neurons had longer dendrites that branched farther from the soma, producing larger dendritic fields. High-speed two-photon calcium imaging of visual responses showed that the orientation preferences of highly tuned PV(+) neurons resembled the preferred orientations of neighboring cells. These results suggest that the diversity of the local neighborhood and the nature of dendritic sampling may both contribute to the response selectivity of PV(+) neurons.
Figures

Similar articles
-
Functional response properties of VIP-expressing inhibitory neurons in mouse visual and auditory cortex.Front Neural Circuits. 2015 May 22;9:22. doi: 10.3389/fncir.2015.00022. eCollection 2015. Front Neural Circuits. 2015. PMID: 26106301 Free PMC article.
-
Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex.Neuron. 2010 Sep 9;67(5):847-57. doi: 10.1016/j.neuron.2010.08.006. Neuron. 2010. PMID: 20826315 Free PMC article.
-
Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency.J Neurosci. 2013 Aug 21;33(34):13713-23. doi: 10.1523/JNEUROSCI.0663-13.2013. J Neurosci. 2013. PMID: 23966693 Free PMC article.
-
Visual representations by cortical somatostatin inhibitory neurons--selective but with weak and delayed responses.J Neurosci. 2010 Oct 27;30(43):14371-9. doi: 10.1523/JNEUROSCI.3248-10.2010. J Neurosci. 2010. PMID: 20980594 Free PMC article.
-
Differential Receptive Field Properties of Parvalbumin and Somatostatin Inhibitory Neurons in Mouse Auditory Cortex.Cereb Cortex. 2015 Jul;25(7):1782-91. doi: 10.1093/cercor/bht417. Epub 2014 Jan 14. Cereb Cortex. 2015. PMID: 24425250 Free PMC article.
Cited by
-
Layer 5 Callosal Parvalbumin-Expressing Neurons: A Distinct Functional Group of GABAergic Neurons.Front Cell Neurosci. 2018 Mar 6;12:53. doi: 10.3389/fncel.2018.00053. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29559891 Free PMC article.
-
Contribution of Innate Cortical Mechanisms to the Maturation of Orientation Selectivity in Parvalbumin Interneurons.J Neurosci. 2017 Jan 25;37(4):820-829. doi: 10.1523/JNEUROSCI.2386-16.2016. J Neurosci. 2017. PMID: 28123018 Free PMC article.
-
Inhibitory Neuron Transplantation into Adult Visual Cortex Creates a New Critical Period that Rescues Impaired Vision.Neuron. 2015 May 20;86(4):1055-1066. doi: 10.1016/j.neuron.2015.03.062. Epub 2015 Apr 30. Neuron. 2015. PMID: 25937171 Free PMC article.
-
Commentary on: Peng, Y et al., Structured recurrent inhibition in the presubiculum could improve information processing.Pflugers Arch. 2021 Nov;473(11):1691-1693. doi: 10.1007/s00424-021-02626-y. Epub 2021 Oct 14. Pflugers Arch. 2021. PMID: 34648053 Free PMC article. No abstract available.
-
Patterned perturbation of inhibition can reveal the dynamical structure of neural processing.Elife. 2020 Feb 19;9:e52757. doi: 10.7554/eLife.52757. Elife. 2020. PMID: 32073400 Free PMC article.
References
-
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
