Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca²⁺ and reactive oxygen species signaling
- PMID: 23843617
- PMCID: PMC3711195
- DOI: 10.1242/jcs.093609
Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca²⁺ and reactive oxygen species signaling
Abstract
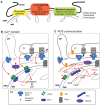
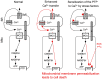
Mitochondria are strategically and dynamically positioned in the cell to spatially coordinate ATP production with energy needs and to allow the local exchange of material with other organelles. Interactions of mitochondria with the sarco-endoplasmic reticulum (SR/ER) have been receiving much attention owing to emerging evidence on the role these sites have in cell signaling, dynamics and biosynthetic pathways. One of the most important physiological and pathophysiological paradigms for SR/ER-mitochondria interactions is in cardiac and skeletal muscle. The contractile activity of these tissues has to be matched by mitochondrial ATP generation that is achieved, at least in part, by propagation of Ca(2+) signals from SR to mitochondria. However, the muscle has a highly ordered structure, providing only limited opportunity for mitochondrial dynamics and interorganellar interactions. This Commentary focuses on the latest advances in the structure, function and disease relevance of the communication between SR/ER and mitochondria in muscle. In particular, we discuss the recent demonstration of SR/ER-mitochondria tethers that are formed by multiple proteins, and local Ca(2+) transfer between SR/ER and mitochondria.
Keywords: Ca2+ signalling; MAM; Mitochondria; Mitochondria-associated membranes; ROS; Ryanodine receptor; Sarcoplasmic reticulum; Uniporter; reactive oxygen species.
Figures


Similar articles
-
SR/ER-mitochondrial local communication: calcium and ROS.Biochim Biophys Acta. 2009 Nov;1787(11):1352-62. doi: 10.1016/j.bbabio.2009.06.004. Epub 2009 Jun 13. Biochim Biophys Acta. 2009. PMID: 19527680 Free PMC article. Review.
-
Quantification of calcium signal transmission from sarco-endoplasmic reticulum to the mitochondria.J Physiol. 2000 Dec 15;529 Pt 3(Pt 3):553-64. doi: 10.1111/j.1469-7793.2000.00553.x. J Physiol. 2000. PMID: 11118489 Free PMC article.
-
Enhanced Mitochondria-SR Tethering Triggers Adaptive Cardiac Muscle Remodeling.Circ Res. 2023 May 26;132(11):e171-e187. doi: 10.1161/CIRCRESAHA.122.321833. Epub 2023 Apr 14. Circ Res. 2023. PMID: 37057625 Free PMC article.
-
Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk.Circ Res. 2012 Sep 14;111(7):863-75. doi: 10.1161/CIRCRESAHA.112.266585. Epub 2012 Jul 9. Circ Res. 2012. PMID: 22777004 Free PMC article.
-
Sarcoplasmic reticulum-mitochondria communication in cardiovascular pathophysiology.Nat Rev Cardiol. 2017 Jun;14(6):342-360. doi: 10.1038/nrcardio.2017.23. Epub 2017 Mar 9. Nat Rev Cardiol. 2017. PMID: 28275246 Review.
Cited by
-
Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice.Aging (Albany NY). 2018 Jan 4;10(1):34-55. doi: 10.18632/aging.101358. Aging (Albany NY). 2018. PMID: 29302020 Free PMC article.
-
Altered Ca(2+) signaling in skeletal muscle fibers of the R6/2 mouse, a model of Huntington's disease.J Gen Physiol. 2014 Nov;144(5):393-413. doi: 10.1085/jgp.201411255. J Gen Physiol. 2014. PMID: 25348412 Free PMC article.
-
Antihypertrophic Effects of Small Molecules that Maintain Mitochondrial ATP Levels Under Hypoxia.EBioMedicine. 2017 Oct;24:147-158. doi: 10.1016/j.ebiom.2017.09.022. Epub 2017 Sep 19. EBioMedicine. 2017. PMID: 28942281 Free PMC article.
-
The Impact of Natriuretic Peptides on Heart Development, Homeostasis, and Disease.Cells. 2024 May 28;13(11):931. doi: 10.3390/cells13110931. Cells. 2024. PMID: 38891063 Free PMC article. Review.
-
Assessing the effects of mitofusin 2 deficiency in the adult heart using 3D electron tomography.Physiol Rep. 2017 Sep;5(17):e13437. doi: 10.14814/phy2.13437. Physiol Rep. 2017. PMID: 28904083 Free PMC article.
References
-
- Andersson D. C., Betzenhauser M. J., Reiken S., Meli A. C., Umanskaya A., Xie W., Shiomi T., Zalk R., Lacampagne A., Marks A. R. (2011). Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 14, 196–207 10.1016/j.cmet.2011.05.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

