Hsp90 inhibitor 17-DMAG decreases expression of conserved herpesvirus protein kinases and reduces virus production in Epstein-Barr virus-infected cells
- PMID: 23843639
- PMCID: PMC3754017
- DOI: 10.1128/JVI.01671-13
Hsp90 inhibitor 17-DMAG decreases expression of conserved herpesvirus protein kinases and reduces virus production in Epstein-Barr virus-infected cells
Abstract
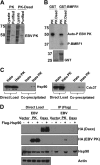
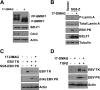
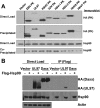
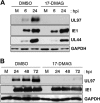
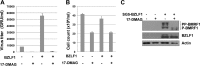
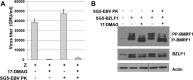
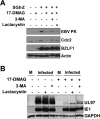
All eight human herpesviruses have a conserved herpesvirus protein kinase (CHPK) that is important for the lytic phase of the viral life cycle. In this study, we show that heat shock protein 90 (Hsp90) interacts directly with each of the eight CHPKs, and we demonstrate that an Hsp90 inhibitor drug, 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), decreases expression of all eight CHPKs in transfected HeLa cells. 17-DMAG also decreases expression the of the endogenous Epstein-Barr virus protein kinase (EBV PK, encoded by the BGLF4 gene) in lytically infected EBV-positive cells and inhibits phosphorylation of several different known EBV PK target proteins. Furthermore, 17-DMAG treatment abrogates expression of the human cytomegalovirus (HCMV) kinase UL97 in HCMV-infected human fibroblasts. Importantly, 17-DMAG treatment decreased the EBV titer approximately 100-fold in lytically infected AGS-Akata cells without causing significant cellular toxicity during the same time frame. Increased EBV PK expression in 17-DMAG-treated AGS-Akata cells did not restore EBV titers, suggesting that 17-DMAG simultaneously targets multiple viral and/or cellular proteins required for efficient viral replication. These results suggest that Hsp90 inhibitors, including 17-DMAG, may be a promising group of drugs that could have profound antiviral effects on herpesviruses.
Figures




Similar articles
-
The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production.J Virol. 2010 May;84(9):4534-42. doi: 10.1128/JVI.02487-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181711 Free PMC article.
-
Curcumin derivative C210 induces Epstein-Barr virus lytic cycle and inhibits virion production by disrupting Hsp90 function.Sci Rep. 2024 Nov 4;14(1):26694. doi: 10.1038/s41598-024-77294-w. Sci Rep. 2024. PMID: 39496752 Free PMC article.
-
Destabilization of PDK1 by Hsp90 inactivation suppresses hepatitis C virus replication through inhibition of PRK2-mediated viral RNA polymerase phosphorylation.Biochem Biophys Res Commun. 2012 Apr 27;421(1):112-8. doi: 10.1016/j.bbrc.2012.03.126. Epub 2012 Apr 3. Biochem Biophys Res Commun. 2012. PMID: 22490666
-
Targeted cancer therapy through 17-DMAG as an Hsp90 inhibitor: Overview and current state of the art.Biomed Pharmacother. 2018 Jun;102:608-617. doi: 10.1016/j.biopha.2018.03.102. Epub 2018 Apr 5. Biomed Pharmacother. 2018. PMID: 29602128 Review.
-
Conserved herpesvirus protein kinases.Biochim Biophys Acta. 2008 Jan;1784(1):203-12. doi: 10.1016/j.bbapap.2007.08.009. Epub 2007 Aug 16. Biochim Biophys Acta. 2008. PMID: 17881303 Free PMC article. Review.
Cited by
-
Role for heat shock protein 90α in the proliferation and migration of HaCaT cells and in the deep second-degree burn wound healing in mice.PLoS One. 2014 Aug 11;9(8):e103723. doi: 10.1371/journal.pone.0103723. eCollection 2014. PLoS One. 2014. PMID: 25111496 Free PMC article.
-
Molecular Determinants for the Inactivation of the Retinoblastoma Tumor Suppressor by the Viral Cyclin-dependent Kinase UL97.J Biol Chem. 2015 Aug 7;290(32):19666-80. doi: 10.1074/jbc.M115.660043. Epub 2015 Jun 21. J Biol Chem. 2015. PMID: 26100623 Free PMC article.
-
Heat Shock Protein 90 Chaperones E1A Early Protein of Adenovirus 5 and Is Essential for Replication of the Virus.Int J Mol Sci. 2021 Feb 18;22(4):2020. doi: 10.3390/ijms22042020. Int J Mol Sci. 2021. PMID: 33670684 Free PMC article.
-
Mechanism of herpesvirus protein kinase UL13 in immune escape and viral replication.Front Immunol. 2022 Nov 30;13:1088690. doi: 10.3389/fimmu.2022.1088690. eCollection 2022. Front Immunol. 2022. PMID: 36531988 Free PMC article. Review.
-
Human Cytomegalovirus Nuclear Capsids Associate with the Core Nuclear Egress Complex and the Viral Protein Kinase pUL97.Viruses. 2018 Jan 13;10(1):35. doi: 10.3390/v10010035. Viruses. 2018. PMID: 29342872 Free PMC article.
References
-
- Roizman B, Knipe DM. 2006. Herpes simplex viruses, p 2502–2601 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA
-
- Cohen J, Straus S, Arvin A. 2006. Varicella-zoster virus replication, pathogenesis, and management, p 2774–2818 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA
-
- Mocarski E, Shenk T, Pass R. 2006. Cytomegaloviruses, p 2702–2772 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA
-
- Yamanashi K, Mori Y, Pellett P. 2006. Human herpesviruses 6 and 7, p 2819–2845 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA
-
- Ganem D. 2006. Kaposi's sarcoma-associated herpesvirus, p 2847–2888 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

