The application of hyaluronic acid-derivatized carbon nanotubes in hematoporphyrin monomethyl ether-based photodynamic therapy for in vivo and in vitro cancer treatment
- PMID: 23843694
- PMCID: PMC3702246
- DOI: 10.2147/IJN.S45407
The application of hyaluronic acid-derivatized carbon nanotubes in hematoporphyrin monomethyl ether-based photodynamic therapy for in vivo and in vitro cancer treatment
Abstract
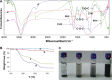
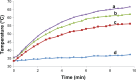
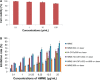
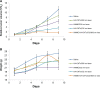
Carbon nanotubes (CNTs) have shown great potential in both photothermal therapy and drug delivery. In this study, a CNT derivative, hyaluronic acid-derivatized CNTs (HA-CNTs) with high aqueous solubility, neutral pH, and tumor-targeting activity, were synthesized and characterized, and then a new photodynamic therapy agent, hematoporphyrin monomethyl ether (HMME), was adsorbed onto the functionalized CNTs to develop HMME-HA-CNTs. Tumor growth inhibition was investigated both in vivo and in vitro by a combination of photothermal therapy and photodynamic therapy using HMME-HA-CNTs. The ability of HMME-HA-CNT nanoparticles to combine local specific photodynamic therapy with external near-infrared photothermal therapy significantly improved the therapeutic efficacy of cancer treatment. Compared with photodynamic therapy or photothermal therapy alone, the combined treatment demonstrated a synergistic effect, resulting in higher therapeutic efficacy without obvious toxic effects to normal organs. Overall, it was demonstrated that HMME-HA-CNTs could be successfully applied to photodynamic therapy and photothermal therapy simultaneously in future tumor therapy.
Keywords: HA-derivatized carbon nanotubes; hematoporphyrin monomethyl ether; photodynamic therapy; photothermal therapy; synergistic effect; tumor targeting.
Figures







Similar articles
-
Effect of PEG-PDLLA polymeric nanovesicles loaded with doxorubicin and hematoporphyrin monomethyl ether on human hepatocellular carcinoma HepG2 cells in vitro.Int J Nanomedicine. 2013;8:4613-22. doi: 10.2147/IJN.S54142. Epub 2013 Dec 2. Int J Nanomedicine. 2013. PMID: 24324333 Free PMC article.
-
Carbon nanotubes for delivery of small molecule drugs.Adv Drug Deliv Rev. 2013 Dec;65(15):1964-2015. doi: 10.1016/j.addr.2013.08.005. Epub 2013 Aug 14. Adv Drug Deliv Rev. 2013. PMID: 23954402 Review.
-
A multi-functional nanoplatform for tumor synergistic phototherapy.Nanotechnology. 2016 Feb 26;27(8):085104. doi: 10.1088/0957-4484/27/8/085104. Epub 2016 Jan 25. Nanotechnology. 2016. PMID: 26808235
-
An intelligent dual stimuli-responsive photosensitizer delivery system with O2-supplying for efficient photodynamic therapy.Colloids Surf B Biointerfaces. 2018 Jul 1;167:299-309. doi: 10.1016/j.colsurfb.2018.04.011. Epub 2018 Apr 10. Colloids Surf B Biointerfaces. 2018. PMID: 29679806
-
Hyaluronic Acid-Conjugated Carbon Nanomaterials for Enhanced Tumour Targeting Ability.Molecules. 2021 Dec 22;27(1):48. doi: 10.3390/molecules27010048. Molecules. 2021. PMID: 35011272 Free PMC article. Review.
Cited by
-
Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment.Molecules. 2020 Nov 14;25(22):5317. doi: 10.3390/molecules25225317. Molecules. 2020. PMID: 33202648 Free PMC article. Review.
-
Carbon nanotubes as cancer therapeutic carriers and mediators.Int J Nanomedicine. 2016 Oct 7;11:5163-5185. doi: 10.2147/IJN.S112660. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27785021 Free PMC article. Review.
-
A study of diagnostic criteria established for two oral mucous diseases by HMME-fluorescence spectroscopy.Lasers Med Sci. 2015 Nov;30(8):2151-6. doi: 10.1007/s10103-015-1776-8. Epub 2015 Jun 13. Lasers Med Sci. 2015. PMID: 26071098
-
Carbon Nanotubes as an Effective Opportunity for Cancer Diagnosis and Treatment.Biosensors (Basel). 2017 Feb 15;7(1):9. doi: 10.3390/bios7010009. Biosensors (Basel). 2017. PMID: 28212271 Free PMC article. Review.
-
Hyaluronic acid-nimesulide conjugates as anticancer drugs against CD44-overexpressing HT-29 colorectal cancer in vitro and in vivo.Int J Nanomedicine. 2017 Mar 27;12:2315-2333. doi: 10.2147/IJN.S120847. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28392690 Free PMC article.
References
-
- Krajcik R, Jung A, Hirsch A, Neuhuber W, Zolk O. Functionalization of carbon nanotubes enables non-covalent binding and intracellular delivery of small interfering RNA for efficient knock-down of genes. Biochem Biophys Res Commun. 2008;369(2):595–602. - PubMed
-
- Chen Z, Pierre D, He H, et al. Adsorption behavior of epirubicin hydrochloride on carboxylated carbon nanotubes. Int J Pharm. 2011;405(1–2):153–161. - PubMed
-
- Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun (Camb) 2004;(1):16–17. - PubMed
-
- Kam NW, Dai H. Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J Am Chem Soc. 2005;127(16):6021–6026. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

