Aqueous Extract of Solanum nigrum Leaves Induces Autophagy and Enhances Cytotoxicity of Cisplatin, Doxorubicin, Docetaxel, and 5-Fluorouracil in Human Colorectal Carcinoma Cells
- PMID: 23843876
- PMCID: PMC3703357
- DOI: 10.1155/2013/514719
Aqueous Extract of Solanum nigrum Leaves Induces Autophagy and Enhances Cytotoxicity of Cisplatin, Doxorubicin, Docetaxel, and 5-Fluorouracil in Human Colorectal Carcinoma Cells
Abstract
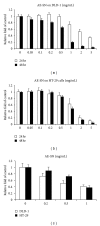
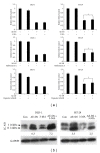
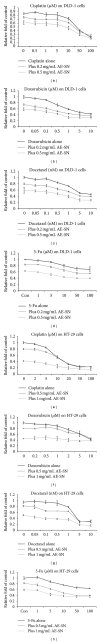
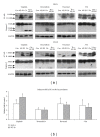
Colorectal cancer is a common cancer worldwide, and chemotherapy is a mainstream approach for advanced and recurrent cases. Development of effective complementary drugs could help improve tumor suppression efficiency and control adverse effects from chemotherapy. The aqueous extract of Solanum nigrum leaves (AE-SN) is an essential component in many traditional Chinese medicine formulas for treating cancer, but there is a lack of evidence verifying its tumor suppression efficacy in colorectal cancer. The purpose of this study is to evaluate the tumor suppression efficacy of AE-SN using DLD-1 and HT-29 human colorectal carcinoma cells and examine the combined drug effect when combined with the chemotherapeutic drugs cisplatin, doxorubicin, docetaxel, and 5-fluorouracil. The results indicated that AE-SN induced autophagy via microtubule-associated protein 1 light chain 3 A/B II accumulation but not caspase-3-dependent apoptosis in both cell lines. The IC50s after 48 hours of treatment were 0.541 and 0.948 mg/ml AE-SN in DLD-1 and HT-29, respectively. AE-SN also demonstrated a combined drug effect with all tested drugs by enhancing cytotoxicity in tumor cells. Our results suggest that AE-SN has potential in the development of complementary chemotherapy for colorectal cancer.
Figures

Similar articles
-
Current development in integrative therapy of traditional Chinese medicine for cancer treatment: A mini-review.J Tradit Complement Med. 2019 Jul 9;10(5):429-433. doi: 10.1016/j.jtcme.2019.07.001. eCollection 2020 Sep. J Tradit Complement Med. 2019. PMID: 32953557 Free PMC article. Review.
-
Cisplatin-, Doxorubicin-, and Docetaxel-Induced Cell Death Promoted by the Aqueous Extract of Solanum nigrum in Human Ovarian Carcinoma Cells.Integr Cancer Ther. 2015 Nov;14(6):546-55. doi: 10.1177/1534735415588826. Epub 2015 Jun 11. Integr Cancer Ther. 2015. PMID: 26069278
-
Aqueous Extract of Solanum nigrum Leaf Activates Autophagic Cell Death and Enhances Docetaxel-Induced Cytotoxicity in Human Endometrial Carcinoma Cells.Evid Based Complement Alternat Med. 2012;2012:859185. doi: 10.1155/2012/859185. Epub 2012 Nov 8. Evid Based Complement Alternat Med. 2012. PMID: 23304219 Free PMC article.
-
Integrated Treatment of Aqueous Extract of Solanum nigrum-Potentiated Cisplatin- and Doxorubicin-Induced Cytotoxicity in Human Hepatocellular Carcinoma Cells.Evid Based Complement Alternat Med. 2015;2015:675270. doi: 10.1155/2015/675270. Epub 2015 Jun 28. Evid Based Complement Alternat Med. 2015. PMID: 26221175 Free PMC article.
-
Paris Polyphylla Inhibits Colorectal Cancer Cells via Inducing Autophagy and Enhancing the Efficacy of Chemotherapeutic Drug Doxorubicin.Molecules. 2019 Jun 3;24(11):2102. doi: 10.3390/molecules24112102. Molecules. 2019. PMID: 31163662 Free PMC article.
Cited by
-
Solasonine Suppresses the Proliferation of Acute Monocytic Leukemia Through the Activation of the AMPK/FOXO3A Axis.Front Oncol. 2021 Jan 29;10:614067. doi: 10.3389/fonc.2020.614067. eCollection 2020. Front Oncol. 2021. PMID: 33585239 Free PMC article.
-
Solanine Attenuates Hepatocarcinoma Migration and Invasion Induced by Acetylcholine.Integr Cancer Ther. 2020 Jan-Dec;19:1534735420909895. doi: 10.1177/1534735420909895. Integr Cancer Ther. 2020. PMID: 32975458 Free PMC article.
-
Current development in integrative therapy of traditional Chinese medicine for cancer treatment: A mini-review.J Tradit Complement Med. 2019 Jul 9;10(5):429-433. doi: 10.1016/j.jtcme.2019.07.001. eCollection 2020 Sep. J Tradit Complement Med. 2019. PMID: 32953557 Free PMC article. Review.
-
Degalactotigonin, a Steroidal Glycoside from Solanum nigrum, Induces Apoptosis and Cell Cycle Arrest via Inhibiting the EGFR Signaling Pathways in Pancreatic Cancer Cells.Biomed Res Int. 2018 Dec 16;2018:3120972. doi: 10.1155/2018/3120972. eCollection 2018. Biomed Res Int. 2018. PMID: 30643798 Free PMC article.
-
Fermented wheat germ extract induced cell death and enhanced cytotoxicity of Cisplatin and 5-Fluorouracil on human hepatocellular carcinoma cells.Evid Based Complement Alternat Med. 2013;2013:121725. doi: 10.1155/2013/121725. Epub 2013 Dec 22. Evid Based Complement Alternat Med. 2013. PMID: 24454483 Free PMC article.
References
-
- Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD) The Lancet Oncology. 2008;9(8):730–756. - PubMed
-
- Cancer Registry Annual Report. Executive Yuan, Taiwan: Department of Health; 2009.
-
- De Dosso S, Sessa C, Saletti P. Adjuvant therapy for colon cancer: present and perspectives. Cancer Treatment Reviews. 2009;35(2):160–166. - PubMed
-
- Kopetz S, Freitas D, Calabrich AFC, Hoff PM. Adjuvant chemotherapy for stage II colon cancer. Oncology. 2008;22(3):260–270. - PubMed
-
- Ernst E, Cassileth BR. The prevalence of complementary/alternative medicine in cancer: a systematic review. Cancer. 1998;83:777–782. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

