Enhancement of efficiency in organic photovoltaic devices containing self-complementary hydrogen-bonding domains
- PMID: 23843901
- PMCID: PMC3701423
- DOI: 10.3762/bjoc.9.122
Enhancement of efficiency in organic photovoltaic devices containing self-complementary hydrogen-bonding domains
Abstract
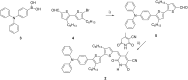
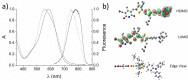
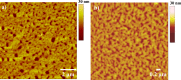
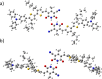
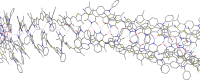
Self-complementary hydrogen-bonding domains were incorporated as the electron deficient unit in "push-pull", p-type small molecules for organic photovoltaic active layers. Such compounds were found to enhance the fill factor, compared with similar non-self-organized compounds reported in the literature, leading to higher device efficiencies. Evidence is presented that the ability of these molecules to form one-dimensional hydrogen-bonded chains and subsequently exhibit hierarchical self-assembly into nanostructured domains can be correlated with improved device efficiency.
Keywords: hydrogen-bonding; organic photovoltaics; self-assembly.
Figures
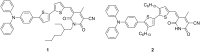

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
