Efficient regio- and stereoselective access to novel fluorinated β-aminocyclohexanecarboxylates
- PMID: 23843909
- PMCID: PMC3701410
- DOI: 10.3762/bjoc.9.130
Efficient regio- and stereoselective access to novel fluorinated β-aminocyclohexanecarboxylates
Abstract
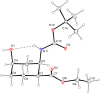
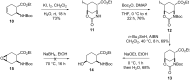
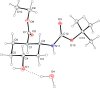
A regio- and stereoselective method has been developed for the synthesis of novel fluorinated 2-aminocyclohexanecarboxylic acid derivatives with the fluorine attached to position 4 of the ring. The synthesis starts from either cis- or trans-β-aminocyclohex-4-enecarboxylic acids and involves regio- and stereoselective transformation of the ring C-C double bond through iodooxazine formation and hydroxylation, followed by hydroxy-fluorine or oxo-fluorine exchange.
Keywords: amino acids; epoxidation; fluorination; hydroxylation; stereoselective reaction.
Figures





References
-
- Gouverneur V, Müller K, editors. Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications. London: Imperial College Press; 2012.
-
- Isanbor C, O’Hagan D. J Fluorine Chem. 2006;127:303. doi: 10.1016/j.jfluchem.2006.01.011. - DOI
-
- Fustero S, Sanz-Cervera J F, Aceña J L, Sánchez-Roselló M. Synlett. 2009:525. doi: 10.1055/s-0028-1087806. - DOI
-
- Kirk K L. J Fluorine Chem. 2006;127:1013. doi: 10.1016/j.jfluchem.2006.06.007. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
