Re-assessment of PrP(Sc) distribution in sporadic and variant CJD
- PMID: 23843953
- PMCID: PMC3700981
- DOI: 10.1371/journal.pone.0066352
Re-assessment of PrP(Sc) distribution in sporadic and variant CJD
Abstract
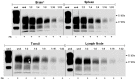
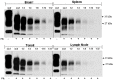
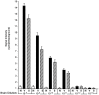
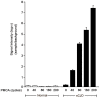
Human prion diseases are fatal neurodegenerative disorders associated with an accumulation of PrP(Sc) in the central nervous system (CNS). Of the human prion diseases, sporadic Creutzfeldt-Jakob disease (sCJD), which has no known origin, is the most common form while variant CJD (vCJD) is an acquired human prion disease reported to differ from other human prion diseases in its neurological, neuropathological, and biochemical phenotype. Peripheral tissue involvement in prion disease, as judged by PrP(Sc) accumulation in the tonsil, spleen, and lymph node has been reported in vCJD as well as several animal models of prion diseases. However, this distribution of PrP(Sc) has not been consistently reported for sCJD. We reexamined CNS and non-CNS tissue distribution and levels of PrP(Sc) in both sCJD and vCJD. Using a sensitive immunoassay, termed SOFIA, we also assessed PrP(Sc) levels in human body fluids from sCJD as well as in vCJD-infected humanized transgenic mice (Tg666). Unexpectedly, the levels of PrP(Sc) in non-CNS human tissues (spleens, lymph nodes, tonsils) from both sCJD and vCJD did not differ significantly and, as expected, were several logs lower than in the brain. Using protein misfolding cyclic amplification (PMCA) followed by SOFIA, PrP(Sc) was detected in cerebrospinal fluid (CSF), but not in urine or blood, in sCJD patients. In addition, using PMCA and SOFIA, we demonstrated that blood from vCJD-infected Tg666 mice showing clinical disease contained prion disease-associated seeding activity although the data was not statistically significant likely due to the limited number of samples examined. These studies provide a comparison of PrP(Sc) in sCJD vs. vCJD as well as analysis of body fluids. Further, these studies also provide circumstantial evidence that in human prion diseases, as in the animal prion diseases, a direct comparison and intraspecies correlation cannot be made between the levels of PrP(Sc) and infectivity.
Conflict of interest statement
Figures


Similar articles
-
Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease.Sci Transl Med. 2017 Nov 22;9(417):eaam7785. doi: 10.1126/scitranslmed.aam7785. Sci Transl Med. 2017. PMID: 29167394 Free PMC article.
-
PMCA Applications for Prion Detection in Peripheral Tissues of Patients with Variant Creutzfeldt-Jakob Disease.Biomolecules. 2020 Mar 5;10(3):405. doi: 10.3390/biom10030405. Biomolecules. 2020. PMID: 32151109 Free PMC article. Review.
-
Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay.Lancet. 2001 Jul 21;358(9277):171-80. doi: 10.1016/s0140-6736(01)05403-4. Lancet. 2001. PMID: 11476832
-
Wide distribution of prion infectivity in the peripheral tissues of vCJD and sCJD patients.Acta Neuropathol. 2021 Mar;141(3):383-397. doi: 10.1007/s00401-021-02270-x. Epub 2021 Feb 2. Acta Neuropathol. 2021. PMID: 33532912 Free PMC article.
-
Neuropathology and molecular biology of variant Creutzfeldt-Jakob disease.Curr Top Microbiol Immunol. 2004;284:133-59. doi: 10.1007/978-3-662-08441-0_6. Curr Top Microbiol Immunol. 2004. PMID: 15148991 Review.
Cited by
-
Prion Strains and Transmission Barrier Phenomena.Pathogens. 2018 Jan 1;7(1):5. doi: 10.3390/pathogens7010005. Pathogens. 2018. PMID: 29301257 Free PMC article. Review.
-
A novel, ultrasensitive assay for tau: potential for assessing traumatic brain injury in tissues and biofluids.J Neurotrauma. 2015 Mar 1;32(5):342-52. doi: 10.1089/neu.2014.3548. Epub 2014 Dec 23. J Neurotrauma. 2015. PMID: 25177776 Free PMC article.
-
Are We Ready for Detecting α-Synuclein Prone to Aggregation in Patients? The Case of "Protein-Misfolding Cyclic Amplification" and "Real-Time Quaking-Induced Conversion" as Diagnostic Tools.Front Neurol. 2018 Jun 6;9:415. doi: 10.3389/fneur.2018.00415. eCollection 2018. Front Neurol. 2018. PMID: 29928254 Free PMC article. Review.
-
Detection of Pathognomonic Biomarker PrPSc and the Contribution of Cell Free-Amplification Techniques to the Diagnosis of Prion Diseases.Biomolecules. 2020 Mar 19;10(3):469. doi: 10.3390/biom10030469. Biomolecules. 2020. PMID: 32204429 Free PMC article. Review.
-
Advanced tests for early and accurate diagnosis of Creutzfeldt-Jakob disease.Nat Rev Neurol. 2016 Jun;12(6):325-33. doi: 10.1038/nrneurol.2016.65. Epub 2016 May 13. Nat Rev Neurol. 2016. PMID: 27174240 Review.
References
-
- Wadsworth JDF, Joiner S, Hill AF, Campbell TA, Desbruslais M, et al. (2001) Tissue distribution of protease resistant prion protein in variant Creutzfeldt–Jakob disease using a highly sensitive immunoblotting assay. Lancet 358: 171–180. - PubMed
-
- Glatzel M, Abela E, Maissen M, Aguzzi A (2003) Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N Engl J Med 349: 1812–1820. - PubMed
-
- Ramasamy I, Law M, Collins S, Brook F (2003) Organ distribution of prion proteins in variant Creutzfeldt–Jakob disease. Lancet Infect Dis 3: 214–222. - PubMed
-
- Cashman NR, Loertscher R, Nalbantoglu J, Shaw I, Kascsak RJ, et al. (1990) Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell 61: 185–192. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

