Intranasally administered antigen 85B gene vaccine in non-replicating human Parainfluenza type 2 virus vector ameliorates mouse atopic dermatitis
- PMID: 23843958
- PMCID: PMC3701015
- DOI: 10.1371/journal.pone.0066614
Intranasally administered antigen 85B gene vaccine in non-replicating human Parainfluenza type 2 virus vector ameliorates mouse atopic dermatitis
Abstract
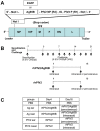
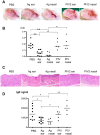
Atopic dermatitis (AD) is a refractory and recurrent inflammatory skin disease. Various factors including heredity, environmental agent, innate and acquired immunity, and skin barrier function participate in the pathogenesis of AD. T -helper (Th) 2-dominant immunological milieu has been suggested in the acute phase of AD. Antigen 85B (Ag85B) is a 30-kDa secretory protein well conserved in Mycobacterium species. Ag85B has strong Th1-type cytokine inducing activity, and is expected to ameliorate Th2 condition in allergic disease. To perform Ag85B function in vivo, effective and less invasive vaccination method is required. Recently, we have established a novel functional virus vector; recombinant human parainfluenza type 2 virus vector (rhPIV2): highly expressive, replication-deficient, and very low-pathogenic vector. In this study, we investigated the efficacy of rhPIV2 engineered to express Ag85B (rhPIV2/Ag85B) in a mouse AD model induced by repeated oxazolone (OX) challenge. Ear swelling, dermal cell infiltrations and serum IgE level were significantly suppressed in the rhPIV2/Ag85B treated mouse group accompanied with elevated IFN-γ and IL-10 mRNA expressions, and suppressed IL-4, TNF-α and MIP-2 mRNA expressions. The treated mice showed no clinical symptom of croup or systemic adverse reactions. The respiratory tract epithelium captured rhPIV2 effectively without remarkable cytotoxic effects. These results suggested that rhPIV2/Ag85B might be a potent therapeutic tool to control allergic disorders.
Conflict of interest statement
Figures



Similar articles
-
Administration of Ag85B showed therapeutic effects to Th2-type cytokine-mediated acute phase atopic dermatitis by inducing regulatory T cells.Arch Dermatol Res. 2009 Feb;301(2):151-7. doi: 10.1007/s00403-008-0873-y. Epub 2008 Jul 17. Arch Dermatol Res. 2009. PMID: 18633632
-
Heat-killed bacillus Calmette-Guérin and Mycobacterium kansasii antigen 85B combined vaccination ameliorates dermatitis in a mouse model of atopic dermatitis by inducing regulatory T cells.Br J Dermatol. 2012 May;166(5):953-63. doi: 10.1111/j.1365-2133.2011.10763.x. Epub 2012 Apr 4. Br J Dermatol. 2012. PMID: 22136598
-
Recombinant Ag85B vaccine by taking advantage of characteristics of human parainfluenza type 2 virus vector showed Mycobacteria-specific immune responses by intranasal immunization.Vaccine. 2014 Mar 26;32(15):1727-35. doi: 10.1016/j.vaccine.2013.11.108. Epub 2014 Jan 29. Vaccine. 2014. PMID: 24486310
-
The role of cytokines/chemokines in the pathogenesis of atopic dermatitis.Curr Probl Dermatol. 2011;41:80-92. doi: 10.1159/000323299. Epub 2011 May 12. Curr Probl Dermatol. 2011. PMID: 21576949 Review.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Immunological association of inducible bronchus-associated lymphoid tissue organogenesis in Ag85B-rHPIV2 vaccine-induced anti-tuberculosis mucosal immune responses in mice.Int Immunol. 2018 Sep 25;30(10):471-481. doi: 10.1093/intimm/dxy046. Int Immunol. 2018. PMID: 30011025 Free PMC article.
References
-
- Grewe M, Bruijnzeel-Koomen CA, Schopf E, Thepen T, Langeveld-Wildschut AG, et al. (1998) A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today 19: 359–361. - PubMed
-
- Kondo H, Ichikawa Y, Imokawa G (1998) Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol 28: 769–779. - PubMed
-
- Ou LS, Goleva E, Hall C, Leung DY (2004) T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol 113: 756–763. - PubMed
-
- Ito Y, Adachi Y, Makino T, Higashiyama H, Fuchizawa T, et al. (2009) Expansion of FOXP3-positive CD4+CD25+ T cells associated with disease activity in atopic dermatitis. Ann Allergy Asthma Immunol 103: 160–165. - PubMed
-
- Yamanaka K, Mizutani H (2011) The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Current problems in dermatology 41: 80–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

