The proton pump inhibitor lansoprazole improves the skeletal phenotype in dystrophin deficient mdx mice
- PMID: 23843959
- PMCID: PMC3699610
- DOI: 10.1371/journal.pone.0066617
The proton pump inhibitor lansoprazole improves the skeletal phenotype in dystrophin deficient mdx mice
Abstract
Background: In Duchenne muscular dystrophy (DMD), loss of the membrane stabilizing protein dystrophin results in myofiber damage. Microinjury to dystrophic myofibers also causes secondary imbalances in sarcolemmic ion permeability and resting membrane potential, which modifies excitation-contraction coupling and increases proinflammatory/apoptotic signaling cascades. Although glucocorticoids remain the standard of care for the treatment of DMD, there is a need to investigate the efficacy of other pharmacological agents targeting the involvement of imbalances in ion flux on dystrophic pathology.
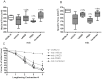
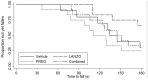
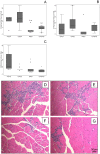
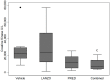
Methodology/principal findings: We designed a preclinical trial to investigate the effects of lansoprazole (LANZO) administration, a proton pump inhibitor, on the dystrophic muscle phenotype in dystrophin deficient (mdx) mice. Eight to ten week-old female mice were assigned to one of four treatment groups (n = 12 per group): (1) vehicle control; (2) 5 mg/kg/day LANZO; (3) 5 mg/kg/day prednisolone; and (4) combined treatment of 5 mg/kg/day prednisolone (PRED) and 5 mg/kg/day LANZO. Treatment was administered orally 5 d/wk for 3 months. At the end of the study, behavioral (Digiscan) and functional outcomes (grip strength and Rotarod) were assessed prior to sacrifice. After sacrifice, body, tissue and organ masses, muscle histology, in vitro muscle force, and creatine kinase levels were measured. Mice in the combined treatment groups displayed significant reductions in the number of degenerating muscle fibers and number of inflammatory foci per muscle field relative to vehicle control. Additionally, mice in the combined treatment group displayed less of a decline in normalized forelimb and hindlimb grip strength and declines in in vitro EDL force after repeated eccentric contractions.
Conclusions/significance: Together our findings suggest that combined treatment of LANZO and prednisolone attenuates some components of dystrophic pathology in mdx mice. Our findings warrant future investigation of the clinical efficacy of LANZO and prednisolone combined treatment regimens in dystrophic pathology.
Conflict of interest statement
Figures
Similar articles
-
Prednisolone attenuates improvement of cardiac and skeletal contractile function and histopathology by lisinopril and spironolactone in the mdx mouse model of Duchenne muscular dystrophy.PLoS One. 2014 Feb 13;9(2):e88360. doi: 10.1371/journal.pone.0088360. eCollection 2014. PLoS One. 2014. PMID: 24551095 Free PMC article.
-
Nutraceutical and pharmaceutical cocktails did not improve muscle function or reduce histological damage in D2-mdx mice.J Appl Physiol (1985). 2019 Oct 1;127(4):1058-1066. doi: 10.1152/japplphysiol.00162.2019. Epub 2019 Jul 11. J Appl Physiol (1985). 2019. PMID: 31295065 Free PMC article.
-
Isometric resistance training increases strength and alters histopathology of dystrophin-deficient mouse skeletal muscle.J Appl Physiol (1985). 2019 Feb 1;126(2):363-375. doi: 10.1152/japplphysiol.00948.2018. Epub 2018 Dec 20. J Appl Physiol (1985). 2019. PMID: 30571283 Free PMC article.
-
Pharmacological control of cellular calcium handling in dystrophic skeletal muscle.Neuromuscul Disord. 2002 Oct;12 Suppl 1:S155-61. doi: 10.1016/s0960-8966(02)00095-0. Neuromuscul Disord. 2002. PMID: 12206810 Review.
-
Mineralocorticoid Receptor Signaling in the Inflammatory Skeletal Muscle Microenvironments of Muscular Dystrophy and Acute Injury.Front Pharmacol. 2022 Jun 28;13:942660. doi: 10.3389/fphar.2022.942660. eCollection 2022. Front Pharmacol. 2022. PMID: 35837290 Free PMC article. Review.
References
-
- Carlson CG (1998) The dystrophinopathies: an alternative to the structural hypothesis. Neurobiology of disease 5: 3–15. - PubMed
-
- Bertorini TE, Bhattacharya SK, Palmieri GM, Chesney CM, Pifer D, et al. (1982) Muscle calcium and magnesium content in Duchenne muscular dystrophy. Neurology 32: 1088–1092. - PubMed
-
- Kerr LM, Sperelakis N (1983) Effects of pH on membrane resistance in normal and dystrophic mouse skeletal muscle fibers. Experimental neurology 82: 203–214. - PubMed
-
- Dunn JF, Bannister N, Kemp GJ, Publicover SJ (1993) Sodium is elevated in mdx muscles: ionic interactions in dystrophic cells. Journal of the neurological sciences 114: 76–80. - PubMed
-
- Sellin LC, Sperelakis N (1978) Decreased potassium permeability in dystrophic mouse skeletal muscle. Experimental neurology 62: 605–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

