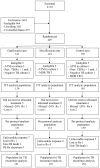
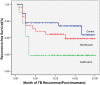
Randomized clinical trial of thrice-weekly 4-month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients
- PMID: 23843980
- PMCID: PMC3700922
- DOI: 10.1371/journal.pone.0067030
Randomized clinical trial of thrice-weekly 4-month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients
Abstract
Background: Shortening tuberculosis (TB) treatment duration is a research priority. This paper presents data from a prematurely terminated randomized clinical trial, of 4-month moxifloxacin or gatifloxacin regimens, in South India.
Methods: Newly diagnosed, sputum-positive HIV-negative pulmonary TB patients were randomly allocated to receive gatifloxacin or moxifloxacin, along with isoniazid and rifampicin for 4 months with pyrazinamide for first 2 months (G or M) or isoniazid and rifampicin for 6 months with ethambutol and pyrazinamide for first 2 months (C). All regimens were administered thrice-weekly. Clinical and bacteriological assessments were done monthly during treatment and for 24 months post-treatment. The Data and Safety Monitoring Board recommended termination of the trial due to high TB recurrence rates in the G and M regimens.
Results: Of 416 patients in intent-to-treat analysis, 6 (5%) of 124, 2 (2%) of 110 and 2 (2%) of 137 patients with drug-susceptible TB in the G, M and C arms respectively had unfavorable response at the end of treatment; during the next 24 months, 17 (15%) of 115, 11 (11%) of 104 and 8 (6%) of 132 patients respectively, had TB recurrence. Of 38 drug-resistant patients 1 of 8 and 3 of 26 in the G and C arms respectively had unfavourable response at the end of treatment; and TB recurrence occurred in 2 of 7 and 2 of 23 patients, respectively. The differences in TB recurrence rates between the G and C arms was statistically significant (p = 0.02). Gastro-intestinal symptoms occurred in 23%, 22% and 9% of patients in the G, M and C arms respectively, but most reactions were mild and manageable with symptomatic measures; 1% required regimen modification.
Conclusions: 4-month thrice-weekly regimens of gatifloxacin or moxifloxacin with isoniazid, rifampicin and pyrazinamide, were inferior to standard 6-month treatment, in patients with newly diagnosed sputum positive pulmonary TB.
Trial registration: Clinical Trials Registry of India CTRI/2012/10/003060.
Conflict of interest statement
Figures
Similar articles
-
Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012918. doi: 10.1002/14651858.CD012918.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828771 Free PMC article.
-
4-month moxifloxacin containing regimens in the treatment of patients with sputum-positive pulmonary tuberculosis in South India - a randomised clinical trial.Trop Med Int Health. 2020 Apr;25(4):483-495. doi: 10.1111/tmi.13371. Epub 2020 Feb 3. Trop Med Int Health. 2020. PMID: 31944502 Clinical Trial.
-
Sputum culture conversion with moxifloxacin-containing regimens in the treatment of patients with newly diagnosed sputum-positive pulmonary tuberculosis in South India.Clin Infect Dis. 2014 Nov 15;59(10):e142-9. doi: 10.1093/cid/ciu550. Epub 2014 Jul 14. Clin Infect Dis. 2014. PMID: 25028463 Clinical Trial.
-
Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial.Lancet. 2009 Apr 4;373(9670):1183-9. doi: 10.1016/S0140-6736(09)60333-0. Lancet. 2009. PMID: 19345831 Free PMC article. Clinical Trial.
-
Fluoroquinolones for the treatment of pulmonary tuberculosis.Drugs. 2007;67(14):2077-99. doi: 10.2165/00003495-200767140-00007. Drugs. 2007. PMID: 17883288 Review.
Cited by
-
Intermittent Versus Daily Pulmonary Tuberculosis Treatment Regimens: A Meta-Analysis.Clin Med Res. 2015 Dec;13(3-4):117-38. doi: 10.3121/cmr.2015.1272. Epub 2015 Jun 8. Clin Med Res. 2015. PMID: 26056374 Free PMC article.
-
Handling the Hurdles on the Way to Anti-tuberculosis Drug Development.Front Chem. 2020 Nov 19;8:586294. doi: 10.3389/fchem.2020.586294. eCollection 2020. Front Chem. 2020. PMID: 33330374 Free PMC article. Review.
-
Assessment of treatment response in tuberculosis.Expert Rev Respir Med. 2016 Jun;10(6):643-54. doi: 10.1586/17476348.2016.1166960. Epub 2016 Mar 31. Expert Rev Respir Med. 2016. PMID: 27030924 Free PMC article. Review.
-
The Role of Fluoroquinolones in the Treatment of Tuberculosis in 2019.Drugs. 2019 Feb;79(2):161-171. doi: 10.1007/s40265-018-1043-y. Drugs. 2019. PMID: 30617959 Free PMC article. Review.
-
Predictors of unfavorable responses to therapy in rifampicin-sensitive pulmonary tuberculosis using an integrated approach of radiological presentation and sputum mycobacterial burden.PLoS One. 2021 Sep 20;16(9):e0257647. doi: 10.1371/journal.pone.0257647. eCollection 2021. PLoS One. 2021. PMID: 34543329 Free PMC article.
References
-
- Fox W, Ellard GA, Mitchison DA (1999) Studies on the treatment of tuberculosis undertaken by the British Medical Research Council Tuberculosis Units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis 3: S231–S279. - PubMed
-
- Tuberculosis Research Centre (2002) Shortening short course chemotherapy: a randomised clinical trial for treatment of smear-positive pulmonary tuberculosis with regimens using ofloxacin in the intensive phase. Ind J Tuberc 49: 27–38.
-
- Sirgel FA, Donald PR, Odhiambo J, Githui W, Umapathy KC, et al. (2000) A multicentre study of the early bactericidal activity of anti- tuberculosis drugs. J Antimicrob Chemother 45: 859–870. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources