Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis
- PMID: 23844014
- PMCID: PMC3701050
- DOI: 10.1371/journal.pone.0067474
Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis
Abstract
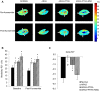
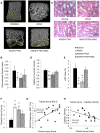
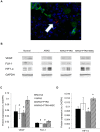
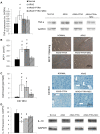
Atherosclerotic renal artery stenosis (ARAS) raises blood pressure and can reduce kidney function. Revascularization of the stenotic renal artery alone does not restore renal medullary structure and function. This study tested the hypothesis that addition of mesenchymal stem cells (MSC) to percutaneous transluminal renal angioplasty (PTRA) can restore stenotic-kidney medullary tubular transport function and attenuate its remodeling. Twenty-seven swine were divided into three ARAS (high-cholesterol diet and renal artery stenosis) and a normal control group. Six weeks after ARAS induction, two groups were treated with PTRA alone or PTRA supplemented with adipose-tissue-derived MSC (10 × 10(6) cells intra-renal). Multi-detector computed tomography and blood-oxygenation-level-dependent (BOLD) MRI studies were performed 4 weeks later to assess kidney hemodynamics and function, and tissue collected a few days later for histology and micro-CT imaging. PTRA effectively decreased blood pressure, yet medullary vascular density remained low. Addition of MSC improved medullary vascularization in ARAS+PTRA+MSC and increased angiogenic signaling, including protein expression of vascular endothelial growth-factor, its receptor (FLK-1), and hypoxia-inducible factor-1α. ARAS+PTRA+MSC also showed attenuated inflammation, although oxidative-stress remained elevated. BOLD-MRI indicated that MSC normalized oxygen-dependent tubular response to furosemide (-4.3 ± 0.9, -0.1 ± 0.4, -1.6 ± 0.9 and -3.6 ± 1.0 s(-1) in Normal, ARAS, ARAS+PTRA and ARAS+PTRA+MSC, respectively, p<0.05), which correlated with a decrease in medullary tubular injury score (R(2) = 0.33, p = 0.02). Therefore, adjunctive MSC delivery in addition to PTRA reduces inflammation, fibrogenesis and vascular remodeling, and restores oxygen-dependent tubular function in the stenotic-kidney medulla, although additional interventions might be required to reduce oxidative-stress. This study supports development of cell-based strategies for renal protection in ARAS.
Conflict of interest statement
Figures

References
-
- de Mast Q, Beutler JJ (2009) The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens 27: 1333–1340. - PubMed
-
- Daghini E, Sanna M, Versari D, Di Paco I, Ghiadoni L, et al. (2010) Long Term Follow up of Hypertensive Patients with Atherosclerosis Renal Artery Stenosis after Percutaneous Renal Artery Angioplasty or Stenting: Blood Pressure Outcome. Journal of Hypertension 28: E210–E210.
-
- Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, et al. (2009) Revascularization versus Medical Therapy for Renal-Artery Stenosis. New England Journal of Medicine 361: 1953–1962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

