Oesophagostomum dentatum extract modulates T cell-dependent immune responses to bystander antigens and prevents the development of allergy in mice
- PMID: 23844022
- PMCID: PMC3699627
- DOI: 10.1371/journal.pone.0067544
Oesophagostomum dentatum extract modulates T cell-dependent immune responses to bystander antigens and prevents the development of allergy in mice
Abstract
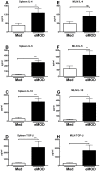
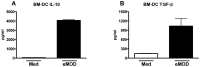
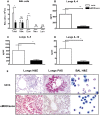
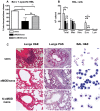
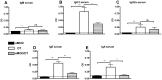
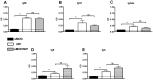
One third of the human population is currently infected by one or more species of parasitic helminths. Certain helminths establish long-term chronic infections resulting in a modulation of the host's immune system with attenuated responsiveness to "bystander" antigens such as allergens or vaccines. In this study we investigated whether parasite-derived products suppress the development of allergic inflammation in a mouse model. We show that extract derived from adult male Oesophagostomum dentatum (eMOD) induced Th2 and regulatory responses in BALB/c mice. Stimulation of bone marrow-derived dendritic cells induced production of regulatory cytokines IL-10 and TGF-beta. In a mouse model of birch pollen allergy, co-administration of eMOD with sensitizing allergen Bet v 1 markedly reduced the production of allergen-specific antibodies in serum as well as IgE-dependent basophil degranulation. Furthermore, eMOD prevented the development of airway inflammation, as demonstrated by attenuation of bronchoalveolar lavages eosinophil influx, peribronchial inflammatory infiltrate, and mucus secretion in lungs and IL-4 and IL-5 levels in lung cell cultures. Reduced secretion of Th2-related cytokines by birch pollen-re-stimulated splenocytes and mesenteric lymph node cells was observed in eMOD-treated/sensitized and challenged mice in comparison to sensitized and challenged controls. The suppressive effects of eMOD were heat-stable. Immunization with model antigens in the presence of eMOD reduced production of antibodies to thymus-dependent but not to thymus-independent antigen, suggesting that suppression of the immune responses by eMOD was mediated by interference with antigen presenting cell or T helper cell function but did not directly suppress B cell function. In conclusion, we have shown that eMOD possesses immunomodulatory properties and that heat-stable factors in eMOD are responsible for the dramatic suppression of allergic responses in a mouse model of type I allergy. The identification and characterization of parasite-derived immune-modulating molecules might have potential for designing novel prophylactic/therapeutic strategies for immune-mediated diseases.
Conflict of interest statement
Figures



Similar articles
-
Prevention of birch pollen-related food allergy by mucosal treatment with multi-allergen-chimers in mice.PLoS One. 2012;7(6):e39409. doi: 10.1371/journal.pone.0039409. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768077 Free PMC article.
-
Oocyst-Derived Extract of Toxoplasma Gondii Serves as Potent Immunomodulator in a Mouse Model of Birch Pollen Allergy.PLoS One. 2016 May 5;11(5):e0155081. doi: 10.1371/journal.pone.0155081. eCollection 2016. PLoS One. 2016. PMID: 27149118 Free PMC article.
-
Direct contact between dendritic cells and bronchial epithelial cells inhibits T cell recall responses towards mite and pollen allergen extracts in vitro.Clin Exp Immunol. 2015 Aug;181(2):207-18. doi: 10.1111/cei.12611. Epub 2015 Jun 11. Clin Exp Immunol. 2015. PMID: 25707463 Free PMC article.
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
-
T regulatory cells and their counterparts: masters of immune regulation.Clin Exp Allergy. 2009 May;39(5):626-39. doi: 10.1111/j.1365-2222.2009.03242.x. Clin Exp Allergy. 2009. PMID: 19422105 Review.
Cited by
-
Human innate lymphoid cells (ILCs) in filarial infections.Parasite Immunol. 2018 Feb;40(2):10.1111/pim.12442. doi: 10.1111/pim.12442. Epub 2017 Jun 15. Parasite Immunol. 2018. PMID: 28504838 Free PMC article. Review.
-
Trichinella spiralis-derived extracellular vesicles induce regulatory T cells and reduce airway allergy in mice.Front Immunol. 2025 Jul 11;16:1637569. doi: 10.3389/fimmu.2025.1637569. eCollection 2025. Front Immunol. 2025. PMID: 40718495 Free PMC article.
-
Safety and long-term immunological effects of CryJ2-LAMP plasmid vaccine in Japanese red cedar atopic subjects: A phase I study.Hum Vaccin Immunother. 2017 Dec 2;13(12):2804-2813. doi: 10.1080/21645515.2017.1329070. Epub 2017 Jun 12. Hum Vaccin Immunother. 2017. PMID: 28605294 Free PMC article. Clinical Trial.
-
The Role of Alveolar Epithelial Type II-Like Cells in Uptake of Structurally Different Antigens and in Polarisation of Local Immune Responses.PLoS One. 2015 Apr 20;10(4):e0124777. doi: 10.1371/journal.pone.0124777. eCollection 2015. PLoS One. 2015. PMID: 25894334 Free PMC article.
-
Toxoplasma gondii tachyzoite-extract acts as a potent immunomodulator against allergic sensitization and airway inflammation.Sci Rep. 2017 Nov 9;7(1):15211. doi: 10.1038/s41598-017-15663-4. Sci Rep. 2017. PMID: 29123241 Free PMC article.
References
-
- Flohr C, Tuyen LN, Lewis S, Quinnell R, Minh TT, et al. (2006) Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: A cross-sectional study. J Allergy Clin Immunol 118: 1305–1311. - PubMed
-
- Rodrigues LC, Newcombe PJ, Cunha SS, Alcantara-Neves NM, Genser B, et al. (2008) Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy 38: 1769–1777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

