Deep vascular imaging in wounds by two-photon fluorescence microscopy
- PMID: 23844028
- PMCID: PMC3699647
- DOI: 10.1371/journal.pone.0067559
Deep vascular imaging in wounds by two-photon fluorescence microscopy
Erratum in
-
Correction: Deep Vascular Imaging in Wounds by Two-Photon Fluorescence Microscopy.PLoS One. 2014 Jan 22;9(1):10.1371/annotation/59bcbe81-eddd-46a4-90dc-88c1ea70df72. doi: 10.1371/annotation/59bcbe81-eddd-46a4-90dc-88c1ea70df72. eCollection 2014. PLoS One. 2014. PMID: 29220845 Free PMC article.
Abstract
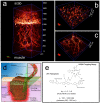
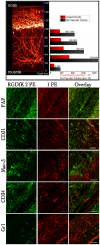
Deep imaging within tissue (over 300 μm) at micrometer resolution has become possible with the advent of two-photon fluorescence microscopy (2PFM). The advantages of 2PFM have been used to interrogate endogenous and exogenous fluorophores in the skin. Herein, we employed the integrin (cell-adhesion proteins expressed by invading angiogenic blood vessels) targeting characteristics of a two-photon absorbing fluorescent probe to image new vasculature and fibroblasts up to ≈ 1600 μm within wound (neodermis)/granulation tissue in lesions made on the skin of mice. Reconstruction revealed three dimensional (3D) architecture of the vascular plexus forming at the regenerating wound tissue and the presence of a fibroblast bed surrounding the capillaries. Biologically crucial events, such as angiogenesis for wound healing, may be illustrated and analyzed in 3D on the whole organ level, providing novel tools for biomedical applications.
Conflict of interest statement
Figures


Similar articles
-
Integrin alpha2beta1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization.J Invest Dermatol. 2007 Feb;127(2):467-78. doi: 10.1038/sj.jid.5700546. Epub 2006 Sep 14. J Invest Dermatol. 2007. PMID: 16977325
-
Human lung fibroblast-derived matrix facilitates vascular morphogenesis in 3D environment and enhances skin wound healing.Acta Biomater. 2017 May;54:333-344. doi: 10.1016/j.actbio.2017.03.035. Epub 2017 Mar 27. Acta Biomater. 2017. PMID: 28351680
-
Temporary angiogenic transformation of the skin graft vasculature after reperfusion.Plast Reconstr Surg. 2010 Jul;126(1):61-70. doi: 10.1097/PRS.0b013e3181da87f6. Plast Reconstr Surg. 2010. PMID: 20595857
-
Angiogenesis and scar formation in healing wounds.Curr Opin Rheumatol. 2013 Jan;25(1):87-91. doi: 10.1097/BOR.0b013e32835b13b6. Curr Opin Rheumatol. 2013. PMID: 23114588 Review.
-
Angiogenesis and wound repair: when enough is enough.J Leukoc Biol. 2016 Nov;100(5):979-984. doi: 10.1189/jlb.4MR0316-102R. Epub 2016 Jul 12. J Leukoc Biol. 2016. PMID: 27406995 Free PMC article. Review.
Cited by
-
Recent developments in vascular imaging techniques in tissue engineering and regenerative medicine.Biomed Res Int. 2015;2015:783983. doi: 10.1155/2015/783983. Epub 2015 Mar 2. Biomed Res Int. 2015. PMID: 25821821 Free PMC article. Review.
-
Scanning darkfield high-resolution microendoscope for label-free microvascular imaging.Biomed Opt Express. 2023 Sep 8;14(10):5097-5112. doi: 10.1364/BOE.498584. eCollection 2023 Oct 1. Biomed Opt Express. 2023. PMID: 37854554 Free PMC article.
-
Novel multimodal MRI and MicroCT imaging approach to quantify angiogenesis and 3D vascular architecture of biomaterials.Sci Rep. 2019 Dec 19;9(1):19474. doi: 10.1038/s41598-019-55411-4. Sci Rep. 2019. PMID: 31857617 Free PMC article.
-
Fluorescent Multifunctional Organic Nanoparticles for Drug Delivery and Bioimaging: A Tutorial Review.Pharmaceutics. 2022 Nov 17;14(11):2498. doi: 10.3390/pharmaceutics14112498. Pharmaceutics. 2022. PMID: 36432688 Free PMC article. Review.
-
Advanced high resolution three-dimensional imaging to visualize the cerebral neurovascular network in stroke.Int J Biol Sci. 2022 Jan 1;18(2):552-571. doi: 10.7150/ijbs.64373. eCollection 2022. Int J Biol Sci. 2022. PMID: 35002509 Free PMC article. Review.
References
-
- Denk W, Svoboda K (1997) Photon upmanship: Why multiphoton imaging is more than a gimmick. Neuron 18: 351–357. - PubMed
-
- Oheim M, Beaurepaire E, Chaigneau E, Mertz J, Charpak S (2001) Two-photon microscopy in brain tissue: Parameters influencing the imaging depth. J Neurosci Methods 111: 29–37. - PubMed
-
- Konig K, Riemann I (2003) High-resolution multiphoton tomography of human skin with subcellular spatial resolution and picosecond time resolution. J Biomed Opt 8: 432–439. - PubMed
-
- Schenke-Layland K, Riemann I, Damour O, Stock UA, Konig K (2006) Two-photon microscopes and in vivo multiphoton tomographs - powerful dicagnostic tools for tissue engineering and drug delivery. Adv Drug Del Rev 58: 878–896. - PubMed
-
- So PTC, Kim H, Kochevar IE (1998) Two-photon deep tissue ex vivo imaging of mouse dermal and subcutaneous structures. Opt Express 3: 339–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

