Comparison of systemic and mucosal immunization with helper-dependent adenoviruses for vaccination against mucosal challenge with SHIV
- PMID: 23844034
- PMCID: PMC3701068
- DOI: 10.1371/journal.pone.0067574
Comparison of systemic and mucosal immunization with helper-dependent adenoviruses for vaccination against mucosal challenge with SHIV
Abstract
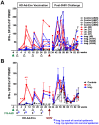
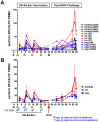
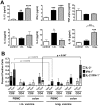
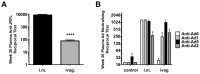
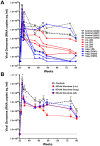
Most HIV-1 infections are thought to occur at mucosal surfaces during sexual contact. It has been hypothesized that vaccines delivered at mucosal surfaces may mediate better protection against HIV-1 than vaccines that are delivered systemically. To test this, rhesus macaques were vaccinated by intramuscular (i.m.) or intravaginal (ivag.) routes with helper-dependent adenoviral (HD-Ad) vectors expressing HIV-1 envelope. Macaques were first immunized intranasally with species C Ad serotype 5 (Ad5) prior to serotype-switching with species C HD-Ad6, Ad1, Ad5, and Ad2 vectors expressing env followed by rectal challenge with CCR5-tropic SHIV-SF162P3. Vaccination by the systemic route generated stronger systemic CD8 T cell responses in PBMC, but weaker mucosal responses. Conversely, mucosal immunization generated stronger CD4 T cell central memory (Tcm) responses in the colon. Intramuscular immunization generated higher levels of env-binding antibodies, but neither produced neutralizing or cytotoxic antibodies. After mucosal SHIV challenge, both groups controlled SHIV better than control animals. However, more animals in the ivag. group had lower viral set points than in in the i.m. group. These data suggest mucosal vaccination may have improve protection against sexually-transmitted HIV. These data also demonstrate that helper-dependent Ad vaccines can mediate robust vaccine responses in the face of prior immunity to Ad5 and during four rounds of adenovirus vaccination.
Conflict of interest statement
Figures

References
-
- Lubeck MD, Natuk R, Myagkikh M, Kalyan N, Aldrich K, et al. (1997) Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med 3: 651–658. - PubMed
-
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, et al. (2002) Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415: 331–335. - PubMed
-
- Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM, et al. (2003) Induction of CD8(+) T Cells to an HIV-1 Antigen through a Prime Boost Regimen with Heterologous E1-Deleted Adenoviral Vaccine Carriers. J Immunol 171: 6774–6779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI065304/AI/NIAID NIH HHS/United States
- AI46969/AI/NIAID NIH HHS/United States
- R01 AI046969/AI/NIAID NIH HHS/United States
- AI42694/AI/NIAID NIH HHS/United States
- R01 HL083047/HL/NHLBI NIH HHS/United States
- AI06795/AI/NIAID NIH HHS/United States
- AI096967/AI/NIAID NIH HHS/United States
- R01 AI096967/AI/NIAID NIH HHS/United States
- HL083047/HL/NHLBI NIH HHS/United States
- DK067324/DK/NIDDK NIH HHS/United States
- R01 AI097241/AI/NIAID NIH HHS/United States
- R01 DK067324/DK/NIDDK NIH HHS/United States
- R01 AI065304/AI/NIAID NIH HHS/United States
- HHSN27201100016C/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

