Intracellular water exchange for measuring the dry mass, water mass and changes in chemical composition of living cells
- PMID: 23844039
- PMCID: PMC3699654
- DOI: 10.1371/journal.pone.0067590
Intracellular water exchange for measuring the dry mass, water mass and changes in chemical composition of living cells
Erratum in
- PLoS One. 2013;8(9). doi:10.1371/annotation/c3a3219b-935b-42ed-b3a7-bbbc36dc1dfe
Abstract
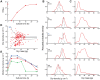
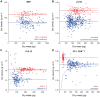
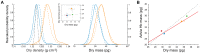
We present a method for direct non-optical quantification of dry mass, dry density and water mass of single living cells in suspension. Dry mass and dry density are obtained simultaneously by measuring a cell's buoyant mass sequentially in an H2O-based fluid and a D2O-based fluid. Rapid exchange of intracellular H2O for D2O renders the cell's water content neutrally buoyant in both measurements, and thus the paired measurements yield the mass and density of the cell's dry material alone. Utilizing this same property of rapid water exchange, we also demonstrate the quantification of intracellular water mass. In a population of E. coli, we paired these measurements to estimate the percent dry weight by mass and volume. We then focused on cellular dry density - the average density of all cellular biomolecules, weighted by their relative abundances. Given that densities vary across biomolecule types (RNA, DNA, protein), we investigated whether we could detect changes in biomolecular composition in bacteria, fungi, and mammalian cells. In E. coli, and S. cerevisiae, dry density increases from stationary to exponential phase, consistent with previously known increases in the RNA/protein ratio from up-regulated ribosome production. For mammalian cells, changes in growth conditions cause substantial shifts in dry density, suggesting concurrent changes in the protein, nucleic acid and lipid content of the cell.
Conflict of interest statement
Figures


References
-
- Potma E, de Boeij WP, van Haastert PJ, Wiersma DA (2001) Real-time visualization of intracellular hydrodynamics in single living cells. Proc Natl Acad Sci USA 98: 1577–1582 doi:10.1073/pnas.031575698 - DOI - PMC - PubMed
-
- Scott M, Gunderson CW, Mateescu EM, Zhang Z, Hwa T (2010) Interdependence of Cell Growth and Gene Expression: Origins and Consequences. Science 330: 1099–1102 doi:10.1126/science.1192588 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

