The bi-functional organization of human basement membranes
- PMID: 23844050
- PMCID: PMC3700973
- DOI: 10.1371/journal.pone.0067660
The bi-functional organization of human basement membranes
Abstract
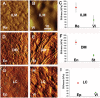
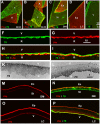
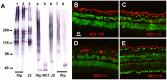
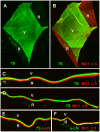
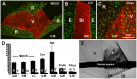
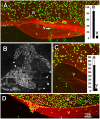
The current basement membrane (BM) model proposes a single-layered extracellular matrix (ECM) sheet that is predominantly composed of laminins, collagen IVs and proteoglycans. The present data show that BM proteins and their domains are asymmetrically organized providing human BMs with side-specific properties: A) isolated human BMs roll up in a side-specific pattern, with the epithelial side facing outward and the stromal side inward. The rolling is independent of the curvature of the tissue from which the BMs were isolated. B) The epithelial side of BMs is twice as stiff as the stromal side, and C) epithelial cells adhere to the epithelial side of BMs only. Side-selective cell adhesion was also confirmed for BMs from mice and from chick embryos. We propose that the bi-functional organization of BMs is an inherent property of BMs and helps build the basic tissue architecture of metazoans with alternating epithelial and connective tissue layers.
Conflict of interest statement
Figures




References
-
- Timpl R (1996) Brown,JC (1996) Supramolecular assembly of basement membranes. Bioassays 18: 123–132. - PubMed
-
- Erickson AC, Couchman JR (2000) Still more complexity in mammalian basement membranes. J Histochem and Cytochem 48: 1291–1306. - PubMed
-
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, et al. (1995) Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev 9: 1896–908. - PubMed
-
- Fassler R, Meyer M (1995) Consequences of lack of β1 integrin gene expression in mice. Genes Dev 9: 1896–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

