Differential expression of three members of the multidomain adhesion CCp family in Babesia bigemina, Babesia bovis and Theileria equi
- PMID: 23844089
- PMCID: PMC3701008
- DOI: 10.1371/journal.pone.0067765
Differential expression of three members of the multidomain adhesion CCp family in Babesia bigemina, Babesia bovis and Theileria equi
Abstract
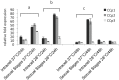
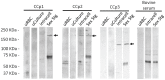
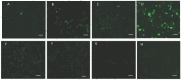
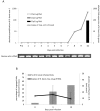
Members of the CCp protein family have been previously described to be expressed on gametocytes of apicomplexan Plasmodium parasites. Knocking out Plasmodium CCp genes blocks the development of the parasite in the mosquito vector, making the CCp proteins potential targets for the development of a transmission-blocking vaccine. Apicomplexans Babesia bovis and Babesia bigemina are the causative agents of bovine babesiosis, and apicomplexan Theileria equi causes equine piroplasmosis. Bovine babesiosis and equine piroplasmosis are the most economically important parasite diseases that affect worldwide cattle and equine industries, respectively. The recent sequencing of the B. bovis and T. equi genomes has provided the opportunity to identify novel genes involved in parasite biology. Here we characterize three members of the CCp family, named CCp1, CCp2 and CCp3, in B. bigemina, B. bovis and T. equi. Using B. bigemina as an in vitro model, expression of all three CCp genes and proteins was demonstrated in temperature-induced sexual stages. Transcripts for all three CCp genes were found in vivo in blood stages of T. equi, and transcripts for CCp3 were detected in vivo in blood stages of B. bovis. However, no protein expression was detected in T. equi blood stages or B. bovis blood stages or B. bovis tick stages. Collectively, the data demonstrated a differential pattern of expression of three orthologous genes of the multidomain adhesion CCp family by B. bigemina, B. bovis and T. equi. The novel CCp members represent potential targets for innovative approaches to control bovine babesiosis and equine piroplasmosis.
Conflict of interest statement
Figures

References
-
- Bishop R, Musoke A, Morzaria S, Gardner M, Nene V (2004) Theileria: intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitol 129: S271–S283. - PubMed
-
- Hunfeld KP, Hildebrandt A, Gray JS (2008) Babesiosis: recent insights into an ancient disease. Int J Parasitol 38: 1219–1237. - PubMed
-
- Mehlhorn H, Schein E (1984) The piroplasm: Life cycle and sexual stages. Adv Parasitol 23: 37–103. - PubMed
-
- Delrieu I, Waller CC, Mota MM, Grainger M, Langhorne J, et al. (2002) PSLAP, a protein with multiple adhesive motifs, is expressed in Plasmodium falciparum gametocytes. Mol Biochem Parasitol 121: 11–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

