Subtle paranodal injury slows impulse conduction in a mathematical model of myelinated axons
- PMID: 23844090
- PMCID: PMC3701069
- DOI: 10.1371/journal.pone.0067767
Subtle paranodal injury slows impulse conduction in a mathematical model of myelinated axons
Abstract
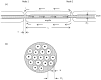
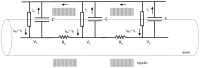
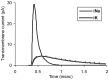
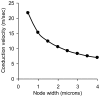
This study explores in detail the functional consequences of subtle retraction and detachment of myelin around the nodes of Ranvier following mild-to-moderate crush or stretch mediated injury. An equivalent electrical circuit model for a series of equally spaced nodes of Ranvier was created incorporating extracellular and axonal resistances, paranodal resistances, nodal capacitances, time varying sodium and potassium currents, and realistic resting and threshold membrane potentials in a myelinated axon segment of 21 successive nodes. Differential equations describing membrane potentials at each nodal region were solved numerically. Subtle injury was simulated by increasing the width of exposed nodal membrane in nodes 8 through 20 of the model. Such injury diminishes action potential amplitude and slows conduction velocity from 19.1 m/sec in the normal region to 7.8 m/sec in the crushed region. Detachment of paranodal myelin, exposing juxtaparanodal potassium channels, decreases conduction velocity further to 6.6 m/sec, an effect that is partially reversible with potassium ion channel blockade. Conduction velocity decreases as node width increases or as paranodal resistance falls. The calculated changes in conduction velocity with subtle paranodal injury agree with experimental observations. Nodes of Ranvier are highly effective but somewhat fragile devices for increasing nerve conduction velocity and decreasing reaction time in vertebrate animals. Their fundamental design limitation is that even small mechanical retractions of myelin from very narrow nodes or slight loosening of paranodal myelin, which are difficult to notice at the light microscopic level of observation, can cause large changes in myelinated nerve conduction velocity.
Conflict of interest statement
Figures

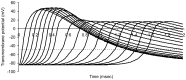

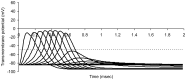
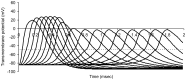
References
-
- ffrench-Constant C, Colognato H, Franklin RJ (2004) Neuroscience. The mysteries of myelin unwrapped. Science 304: 688–689. - PubMed
-
- Waxman SG (2006) Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci 7: 932–941. - PubMed
-
- Nashmi R, Fehlings MG (2001) Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Brain Res Rev 38: 165–191. - PubMed
-
- Boron WF, Boulpaep EL (2005) Medical Physiology. Philadelphia: Elsevier. 1319 p.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

