MHC class I antigen presentation of DRiP-derived peptides from a model antigen is not dependent on the AAA ATPase p97
- PMID: 23844095
- PMCID: PMC3699533
- DOI: 10.1371/journal.pone.0067796
MHC class I antigen presentation of DRiP-derived peptides from a model antigen is not dependent on the AAA ATPase p97
Abstract
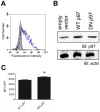
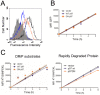
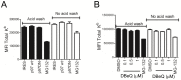
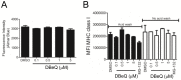
CD8(+) T cells are responsible for killing cells of the body that have become infected or oncogenically transformed. In order to do so, effector CD8(+) T cells must recognize their cognate antigenic peptide bound to a MHC class I molecule that has been directly presented by the target cell. Due to the rapid nature of antigen presentation, it is believed that antigenic peptides are derived from a subset of newly synthesized proteins which are degraded almost immediately following synthesis and termed Defective Ribosomal Products or DRiPs. We have recently reported on a bioassay which can distinguish antigen presentation of DRiP substrates from other forms of rapidly degraded proteins and found that poly-ubiquitin chain disassembly may be necessary for efficient DRiP presentation. The AAA ATPase p97 protein is necessary for efficient cross-presentation of antigens on MHC class I molecules and plays an important role in extracting mis-folded proteins from the endoplasmic reticulum. Here, we find that genetic ablation or chemical inhibition of p97 does not diminish DRiP antigen presentation to any great extent nor does it alter the levels of MHC class I molecules on the cell surface, despite our observations that p97 inhibition increased the levels of poly-ubiquitinated proteins in the cell. These data demonstrate that inhibiting poly-ubiquitin chain disassembly alone is insufficient to abolish DRiP presentation.
Conflict of interest statement
Figures


References
-
- Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, et al. (2001) Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. Journal of Immunology 166: 6099–6103. - PubMed
-
- Larsson M, Fonteneau JF, Somersan S, Sanders C, Bickham K, et al. (2001) Efficiency of cross presentation of vaccinia virus-derived antigens by human dendritic cells. Eur J Immunol 31: 3432–3442. - PubMed
-
- Khan S, de Giuli R, Schmidtke G, Bruns M, Buchmeier M, et al. (2001) Cutting edge: neosynthesis is required for the presentation of a T cell epitope from a long-lived viral protein. J Immunol 167: 4801–4804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

