Intracranial aneurysm formation in type-one diabetes rats
- PMID: 23844137
- PMCID: PMC3699459
- DOI: 10.1371/journal.pone.0067949
Intracranial aneurysm formation in type-one diabetes rats
Abstract
Background & objective: Diabetes mellitus (DM) plays an important role in the pathogenesis of vascular complications including arteriosclerosis and ischemic stroke. Whether DM impacts intracranial aneurysm (IA) formation has not been extensively investigated. In this study, we tested the underlying mechanism of type one DM (T1DM) induced IA formation in rats.
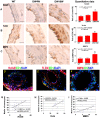
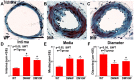
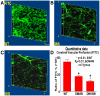
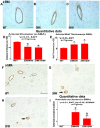
Experimental approaches: T1DM was induced by streptozotocin injection. Rats were euthanized at 0, 4 and 10 weeks after T1DM induction. To evaluate cerebral vascular perfusion, Fluorescein isothiocyanate - dye was injected at 5 min prior to euthanasia. Vascular perfusion was measured by laser scanning confocal microscopy. Trichrome, Elastica van Gieson, alpha-smooth muscle actin (a-SMA) and receptor of advanced glycation end-products (RAGE), toll-like receptor 4 (TLR4) and matrix metalloproteinase 9 (MMP9) immunostaining were performed. The IA formation was classified by 0-3 stages: 0: Normal; 1: Endothelial damage; 2: Moderate protrusion; and 3: Saccular aneurysm formation.
Results: T1DM significantly increased IA formation identified by the classification of aneurysmal changes compared with non-DM rats (p<0.05). However, T1DM induced IA formations were classified as stage 1 and stage 2, but not stage 3. Cerebral vascular perfusion was significantly decreased in T1DM rats compared to non-DM rats (p<0.01). DM10W rats exhibited a significant decrease of cerebral vascular perfusion compared to DM4W rats (p<0.05). T1DM rats also significantly increased the internal carotid artery (ICA) intimae and media thickness, and decreased the internal carotid artery diameter compared to non-DM rats. RAGE, MMP9 and TLR4 expression were significantly increased in T1DM rats compared to non-DM rats. The increased RAGE, TLR4 and MMP9 significantly correlated with IA formation (p<0.05).
Conclusion: T1DM increases IA formation. The increased RAGE, MMP9 and TLR4 expressions might contribute to IA formation in T1DM rats.
Conflict of interest statement
Figures

References
-
- Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, et al. (2004) Trends in cardiovascular complications of diabetes. JAMA 292: 2495–2499. - PubMed
-
- Tamura T, Jamous MA, Kitazato KT, Yagi K, Tada Y, et al. (2009) Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. J Hypertens 27: 1284–1292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

