Anti-aggregating effect of the naturally occurring dipeptide carnosine on aβ1-42 fibril formation
- PMID: 23844165
- PMCID: PMC3700870
- DOI: 10.1371/journal.pone.0068159
Anti-aggregating effect of the naturally occurring dipeptide carnosine on aβ1-42 fibril formation
Abstract
Carnosine is an endogenous dipeptide abundant in the central nervous system, where by acting as intracellular pH buffering molecule, Zn/Cu ion chelator, antioxidant and anti-crosslinking agent, it exerts a well-recognized multi-protective homeostatic function for neuronal and non-neuronal cells. Carnosine seems to counteract proteotoxicity and protein accumulation in neurodegenerative conditions, such as Alzheimer's Disease (AD). However, its direct impact on the dynamics of AD-related fibril formation remains uninvestigated. We considered the effects of carnosine on the formation of fibrils/aggregates of the amyloidogenic peptide fragment Aβ1-42, a major hallmark of AD injury. Atomic force microscopy and thioflavin T assays showed inhibition of Aβ1-42 fibrillogenesis in vitro and differences in the aggregation state of Aβ1-42 small pre-fibrillar structures (monomers and small oligomers) in the presence of carnosine. in silico molecular docking supported the experimental data, calculating possible conformational carnosine/Aβ1-42 interactions. Overall, our results suggest an effective role of carnosine against Aβ1-42 aggregation.
Conflict of interest statement
Figures
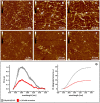

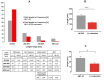
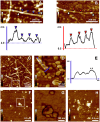
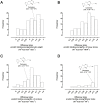
References
-
- Hipkiss AR (2009) Carnosine and its possible roles in nutrition and health. Adv Food Nutr Res 57: 87–154. - PubMed
-
- Hoffmann AM, Bakardjiev A, Bauer K (1996) Carnosine-synthesis in cultures of rat glial cells is restricted to oligodendrocytes and carnosine uptake to astrocytes. Neurosci Lett 215: 29–32. - PubMed
-
- De Marchis S, Melcangi RC, Modena C, Cavaretta I, Peretto P, et al. (1997) Identification of the glial cell types containing carnosine-related peptides in the rat brain. Neurosci Lett 237: 37–40. - PubMed
-
- Bakardjiev A (1998) Carnosine and beta-alanine release is stimulated by glutamatergic receptors in cultured rat oligodendrocytes. Glia 24: 346–351. - PubMed
-
- Bakardjiev A, Bauer K (2000) Biosynthesis, release, and uptake of carnosine in primary cultures. Biochemistry (Mosc) 65: 779–782. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

