Development of low phytate rice by RNAi mediated seed-specific silencing of inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene (IPK1)
- PMID: 23844166
- PMCID: PMC3699528
- DOI: 10.1371/journal.pone.0068161
Development of low phytate rice by RNAi mediated seed-specific silencing of inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene (IPK1)
Abstract
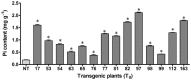
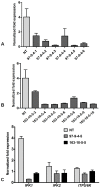
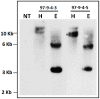
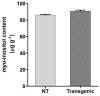
Phytic acid (InsP(6)) is considered to be the major source of phosphorus and inositol phosphates in most cereal grains. However, InsP(6) is not utilized efficiently by monogastric animals due to lack of phytase enzyme. Furthermore, due to its ability to chelate mineral cations, phytic acid is considered to be an antinutrient that renders these minerals unavailable for absorption. In view of these facts, reducing the phytic acid content in cereal grains is a desired goal for the genetic improvement of several crops. In the present study, we report the RNAi-mediated seed-specific silencing (using the Oleosin18 promoter) of the IPK1 gene, which catalyzes the last step of phytic acid biosynthesis in rice. The presence of the transgene cassette in the resulting transgenic plants was confirmed by molecular analysis, indicating the stable integration of the transgene. The subsequent T4 transgenic seeds revealed 3.85-fold down-regulation in IPK1 transcripts, which correlated to a significant reduction in phytate levels and a concomitant increase in the amount of inorganic phosphate (Pi). The low-phytate rice seeds also accumulated 1.8-fold more iron in the endosperm due to the decreased phytic acid levels. No negative effects were observed on seed germination or in any of the agronomic traits examined. The results provide evidence that silencing of IPK1 gene can mediate a substantial reduction in seed phytate levels without hampering the growth and development of transgenic rice plants.
Conflict of interest statement
Figures

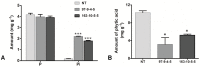


References
-
- Holm PB, Kristiansen KN, Pedersen HB (2002) Transgenic approaches in commonly consumed cereals to improve iron and zinc content and bioavailability. J Nutr 132: 514–516. - PubMed
-
- Kuwano M, Ohyama A, Tanaka Y, Mimura T, Takaiwa F, et al. (2006) Molecular breeding for transgenic rice with low phytic acid phenotype through manipulating myo inositol 3 phosphate synthase gene. Mol Breeding 18: 263–272.
-
- Raboy V (2009) Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci 177: 281–296.
-
- Lucca P, Hurrell R, Potrykus I (2001) Genetic engineering approaches to improve the bioavailability and the level of iron in rice grains. Theor Appl Genet 102: 392–397.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

