TLR-2/TLR-4 TREM-1 signaling pathway is dispensable in inflammatory myeloid cells during sterile kidney injury
- PMID: 23844229
- PMCID: PMC3700949
- DOI: 10.1371/journal.pone.0068640
TLR-2/TLR-4 TREM-1 signaling pathway is dispensable in inflammatory myeloid cells during sterile kidney injury
Abstract
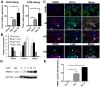
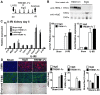
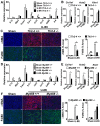
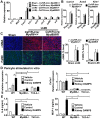
Inflammatory macrophages are abundant in kidney disease, stimulating repair, or driving chronic inflammation and fibrosis. Damage associated molecules (DAMPs), released from injured cells engage pattern recognition receptors (PRRs) on macrophages, contributing to activation. Understanding mechanisms of macrophage activation during kidney injury may lead to strategies to alleviate chronic disease. We identified Triggering-Receptor-in-Myeloid-cells (TREM)-1, a regulator of TLR signaling, as highly upregulated in kidney inflammatory macrophages and tested the roles of these receptors in macrophage activation and kidney disease. Kidney DAMPs activated macrophages in vitro, independently of TREM-1, but partially dependent on TLR-2/-4, MyD88. In two models of progressive interstitial kidney disease, TREM-1 blockade had no impact on disease or macrophage activation in vivo, but TLR-2/-4, or MyD88 deficiency was anti-inflammatory and anti-fibrotic. When MyD88 was mutated only in the myeloid lineage, however, there was no bearing on macrophage activation or disease progression. Instead, TLR-2/-4 or MyD88 deficiency reduced activation of mesenchyme lineage cells resulting in reduced inflammation and fibrosis, indicating that these pathways play dominant roles in activation of myofibroblasts but not macrophages. To conclude, TREM-1, TLR2/4 and MyD88 signaling pathways are redundant in myeloid cell activation in kidney injury, but the latter appear to regulate activation of mesenchymal cells.
Conflict of interest statement
Figures

References
-
- Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, et al. (2012) The renal mononuclear phagocytic system. Journal of the American Society of Nephrology 23: 194–203 doi:10.1681/ASN.2011070680 - DOI - PMC - PubMed
-
- Lin SL, Castano AP, Nowlin BT, Lupher MLJ, Duffield JS (2009) Bone Marrow Ly6Chigh Monocytes Are Selectively Recruited to Injured Kidney and Differentiate into Functionally Distinct Populations. The Journal of Immunology 183: 6733–6743. - PubMed
-
- Anders HJ (2010) Toll-like receptors and danger signaling in kidney injury. Journal of the American Society of Nephrology 21: 1270–1274 doi:10.1681/ASN.2010030233 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK093493/DK/NIDDK NIH HHS/United States
- AI 073441/AI/NIAID NIH HHS/United States
- R01 HL086883/HL/NHLBI NIH HHS/United States
- HL086883/HL/NHLBI NIH HHS/United States
- DK84077/DK/NIDDK NIH HHS/United States
- R01 DK084077/DK/NIDDK NIH HHS/United States
- R24 DK094768/DK/NIDDK NIH HHS/United States
- R01 AI081948/AI/NIAID NIH HHS/United States
- DK87389/DK/NIDDK NIH HHS/United States
- RC1 DK087389/DK/NIDDK NIH HHS/United States
- DK93493/DK/NIDDK NIH HHS/United States
- DK94768/DK/NIDDK NIH HHS/United States
- R01 DK094933/DK/NIDDK NIH HHS/United States
- R01 AI073441/AI/NIAID NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

