C-Jun N-terminal kinase (JNK) mediates Wnt5a-induced cell motility dependent or independent of RhoA pathway in human dental papilla cells
- PMID: 23844260
- PMCID: PMC3700942
- DOI: 10.1371/journal.pone.0069440
C-Jun N-terminal kinase (JNK) mediates Wnt5a-induced cell motility dependent or independent of RhoA pathway in human dental papilla cells
Abstract
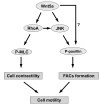
Wnt5a plays an essential role in tissue development by regulating cell migration, though the molecular mechanisms are still not fully understood. Our study investigated the pathways involved in Wnt5a-dependent cell motility during the formation of dentin and pulp. Over-expression of Wnt5a promoted cell adhesion and formation of focal adhesion complexes (FACs) in human dental papilla cells (hDPCs), while inhibiting cell migration. Instead of activating the canonical Wnt signal pathway in hDPCs, Wnt5a stimulation induced activation of the JNK signal in a RhoA-dependent or independent manner. Inhibiting JNK abrogated Wnt5a-induced FACs formation but not cytoskeletal rearrangement. Both dominant negative RhoA (RhoA T19N) and constitutively active RhoA mutants (RhoA Q63L) blocked the Wnt5a-dependent changes in hDPCs adhesion, migration and cytoskeletal rearrangement here too, with the exception of the formation of FACs. Taken together, our study suggested that RhoA and JNK signaling have roles in mediating Wnt5a-dependent adhesion and migration in hDPCs, and the Wnt5a/JNK pathway acts both dependently and independently of the RhoA pathway.
Conflict of interest statement
Figures





Similar articles
-
PI3K/Akt-dependent phosphorylation of GSK3β and activation of RhoA regulate Wnt5a-induced gastric cancer cell migration.Cell Signal. 2013 Feb;25(2):447-56. doi: 10.1016/j.cellsig.2012.10.012. Epub 2012 Oct 31. Cell Signal. 2013. PMID: 23123500
-
C-Jun N-terminal kinase (JNK) pathway activation is essential for dental papilla cells polarization.PLoS One. 2021 Mar 26;16(3):e0233944. doi: 10.1371/journal.pone.0233944. eCollection 2021. PLoS One. 2021. PMID: 33770099 Free PMC article.
-
WNT5A inhibits human dental papilla cell proliferation and migration.Biochem Biophys Res Commun. 2009 Dec 18;390(3):1072-8. doi: 10.1016/j.bbrc.2009.10.136. Epub 2009 Oct 28. Biochem Biophys Res Commun. 2009. PMID: 19878652
-
Vertebrate Wnt5a - At the crossroads of cellular signalling.Semin Cell Dev Biol. 2022 May;125:3-10. doi: 10.1016/j.semcdb.2021.10.002. Epub 2021 Oct 20. Semin Cell Dev Biol. 2022. PMID: 34686423 Review.
-
WNT5A in tumor development and progression: A comprehensive review.Biomed Pharmacother. 2022 Nov;155:113599. doi: 10.1016/j.biopha.2022.113599. Epub 2022 Sep 9. Biomed Pharmacother. 2022. PMID: 36089446 Review.
Cited by
-
Casein kinase 1 epsilon (CK1ε) as a potential therapeutic target in chronic liver disease.J Vet Sci. 2025 May;26(3):e30. doi: 10.4142/jvs.24321. J Vet Sci. 2025. PMID: 40461423 Free PMC article. Review.
-
Non-canonical WNT5A signaling up-regulates the expression of the tumor suppressor 15-PGDH and induces differentiation of colon cancer cells.Mol Oncol. 2016 Nov;10(9):1415-1429. doi: 10.1016/j.molonc.2016.07.011. Epub 2016 Aug 1. Mol Oncol. 2016. PMID: 27522468 Free PMC article.
-
DAAM1 and DAAM2 are co-required for myocardial maturation and sarcomere assembly.Dev Biol. 2015 Dec 1;408(1):126-39. doi: 10.1016/j.ydbio.2015.10.003. Epub 2015 Oct 23. Dev Biol. 2015. PMID: 26526197 Free PMC article.
-
The role of Atg5 gene in tumorigenesis under autophagy deficiency conditions.Kaohsiung J Med Sci. 2024 Jul;40(7):631-641. doi: 10.1002/kjm2.12853. Epub 2024 Jun 3. Kaohsiung J Med Sci. 2024. PMID: 38826147 Free PMC article.
-
Expression patterns of WNT/β-CATENIN signaling molecules during human tooth development.J Mol Histol. 2014 Oct;45(5):487-96. doi: 10.1007/s10735-014-9572-5. Epub 2014 Mar 20. J Mol Histol. 2014. PMID: 24647585
References
-
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH et al. (2003) Cell migration: integrating signals from front to back. Science 302: 1704–1709. doi:10.1126/science.1092053. PubMed: 14657486. - DOI - PubMed
-
- Vicente-Manzanares M, Horwitz AR (2011) Adhesion dynamics at a glance. J Cell Sci 124: 3923-3927. doi:10.1242/jcs.095653. PubMed: 22194302. - DOI - PMC - PubMed
-
- Aman A, Piotrowski T (2010) Cell migration during morphogenesis. Dev Biol 341: 20-33. doi:10.1016/j.ydbio.2009.11.014. PubMed: 19914236. - DOI - PubMed
-
- Thesleff I, Hurmerinta K (1981) Tissue interactions in tooth development. Differentiation 18: 75-88. doi:10.1111/j.1432-0436.1981.tb01107.x. PubMed: 7011890. - DOI - PubMed
-
- Thesleff I, Vaahtokari A, Vainio S, Jowett A (1996) Molecular mechanisms of cell and tissue interactions during early tooth development. Anat Rec 245: 151-161. doi:10.1002/(SICI)1097-0185(199606)245:2. PubMed: 8769660. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

