Adhoc electromagnetic compatibility testing of non-implantable medical devices and radio frequency identification
- PMID: 23845013
- PMCID: PMC3716957
- DOI: 10.1186/1475-925X-12-71
Adhoc electromagnetic compatibility testing of non-implantable medical devices and radio frequency identification
Abstract
Background: The use of radiofrequency identification (RFID) in healthcare is increasing and concerns for electromagnetic compatibility (EMC) pose one of the biggest obstacles for widespread adoption. Numerous studies have documented that RFID can interfere with medical devices. The majority of past studies have concentrated on implantable medical devices such as implantable pacemakers and implantable cardioverter defibrillators (ICDs). This study examined EMC between RFID systems and non-implantable medical devices.
Methods: Medical devices were exposed to 19 different RFID readers and one RFID active tag. The RFID systems used covered 5 different frequency bands: 125-134 kHz (low frequency (LF)); 13.56 MHz (high frequency (HF)); 433 MHz; 915 MHz (ultra high frequency (UHF])) and 2.4 GHz. We tested three syringe pumps, three infusion pumps, four automatic external defibrillators (AEDs), and one ventilator. The testing procedure is modified from American National Standards Institute (ANSI) C63.18, Recommended Practice for an On-Site, Ad Hoc Test Method for Estimating Radiated Electromagnetic Immunity of Medical Devices to Specific Radio-Frequency Transmitters.
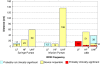
Results: For syringe pumps, we observed electromagnetic interference (EMI) during 13 of 60 experiments (22%) at a maximum distance of 59 cm. For infusion pumps, we observed EMI during 10 of 60 experiments (17%) at a maximum distance of 136 cm. For AEDs, we observed EMI during 18 of 75 experiments (24%) at a maximum distance of 51 cm. The majority of the EMI observed was classified as probably clinically significant or left the device inoperable. No EMI was observed for all medical devices tested during exposure to 433 MHz (two readers, one active tag) or 2.4 GHz RFID (two readers).
Conclusion: Testing confirms that RFID has the ability to interfere with critical medical equipment. Hospital staff should be aware of the potential for medical device EMI caused by RFID systems and should be encouraged to perform on-site RF immunity tests prior to RFID system deployment or prior to placing new medical devices in an RFID environment. The methods presented in this paper are time-consuming and burdensome and suggest the need for standard test methods for assessing the immunity of medical devices to RFID systems.
Figures


Similar articles
-
Feasibility results of an electromagnetic compatibility test protocol to evaluate medical devices to radio frequency identification exposure.Biomed Eng Online. 2014 Aug 3;13:110. doi: 10.1186/1475-925X-13-110. Biomed Eng Online. 2014. PMID: 25086451 Free PMC article.
-
In vitro tests reveal sample radiofrequency identification readers inducing clinically significant electromagnetic interference to implantable pacemakers and implantable cardioverter-defibrillators.Heart Rhythm. 2010 Jan;7(1):99-107. doi: 10.1016/j.hrthm.2009.09.071. Epub 2009 Oct 12. Heart Rhythm. 2010. PMID: 20129290
-
Electromagnetic interference from radio frequency identification inducing potentially hazardous incidents in critical care medical equipment.JAMA. 2008 Jun 25;299(24):2884-90. doi: 10.1001/jama.299.24.2884. JAMA. 2008. PMID: 18577733
-
Radiofrequency identification and medical devices: the regulatory framework on electromagnetic compatibility. Part II: active implantable medical devices.Expert Rev Med Devices. 2012 May;9(3):289-97. doi: 10.1586/erd.12.5. Expert Rev Med Devices. 2012. PMID: 22702260 Review.
-
Radiofrequency identification and medical devices: the regulatory framework on electromagnetic compatibility. Part I: medical devices.Expert Rev Med Devices. 2012 May;9(3):283-8. doi: 10.1586/erd.12.4. Expert Rev Med Devices. 2012. PMID: 22702259 Review.
Cited by
-
Feasibility results of an electromagnetic compatibility test protocol to evaluate medical devices to radio frequency identification exposure.Biomed Eng Online. 2014 Aug 3;13:110. doi: 10.1186/1475-925X-13-110. Biomed Eng Online. 2014. PMID: 25086451 Free PMC article.
-
Application safety evaluation of the radio frequency identification tag under magnetic resonance imaging.Biomed Eng Online. 2014 Sep 4;13:129. doi: 10.1186/1475-925X-13-129. Biomed Eng Online. 2014. PMID: 25187420 Free PMC article.
-
Extremely Low-Frequency Electromagnetic Fields Increase the Expression of Anagen-Related Molecules in Human Dermal Papilla Cells via GSK-3β/ERK/Akt Signaling Pathway.Int J Mol Sci. 2020 Jan 25;21(3):784. doi: 10.3390/ijms21030784. Int J Mol Sci. 2020. PMID: 31991762 Free PMC article.
-
Workers with Cardiac AIMD Exposed to EMF: Methods and Case Studies for Risk Analysis in the Framework of the European Regulations.Int J Environ Res Public Health. 2021 Sep 15;18(18):9709. doi: 10.3390/ijerph18189709. Int J Environ Res Public Health. 2021. PMID: 34574648 Free PMC article.
References
-
- Seidman SJ, Brockman R, Lewis BM, Guag J, Shein MJ, Clement WJ, Kippola J, Digby D, Barber C, Huntwork D. In vitro tests reveal sample radiofrequency identification readers inducing clinically significant electromagnetic interference to implantable pacemakers and implantable cardioverter-defibrillators. Hear Rhythm. 2010;7:99–107. doi: 10.1016/j.hrthm.2009.09.071. - DOI - PubMed
-
- Van der Togt R, Van Lieshout EJ, Hensbroek R, Beinat E, Binnekade JM, Bakker PJM. Electromagnetic interference from radio frequency identification inducing potentially hazardous incidents in critical care medical equipment. Jama-J Am Med Assoc. 2008;299:2884–2890. doi: 10.1001/jama.299.24.2884. - DOI - PubMed
-
- Futatsumori S, Hikage T, Nojima T, Koike B, Fujimoto H, Toyoshima T. A Novel Assessment Methodology for the EMI Occurrence in Implantable Medical Devices Based Upon Magnetic Flux Distribution of RFID Reader/writers. Electromagnetic Compatibility, 2007 EMC 2007 IEEE International Symposium on; 9–13 July 2007. 2007. pp. 1–6.
-
- Ogirala A, Stachel JR, Mickle MH. Electromagnetic Interference of Cardiac Rhythmic Monitoring Devices to Radio Frequency Identification: Analytical Analysis and Mitigation Methodology. Inf Technol Biomed, IEEE Trans. 2011;15:848–853. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

