How protein targeting to primary plastids via the endomembrane system could have evolved? A new hypothesis based on phylogenetic studies
- PMID: 23845039
- PMCID: PMC3716720
- DOI: 10.1186/1745-6150-8-18
How protein targeting to primary plastids via the endomembrane system could have evolved? A new hypothesis based on phylogenetic studies
Abstract
Background: It is commonly assumed that a heterotrophic ancestor of the supergroup Archaeplastida/Plantae engulfed a cyanobacterium that was transformed into a primary plastid; however, it is still unclear how nuclear-encoded proteins initially were imported into the new organelle. Most proteins targeted to primary plastids carry a transit peptide and are transported post-translationally using Toc and Tic translocons. There are, however, several proteins with N-terminal signal peptides that are directed to higher plant plastids in vesicles derived from the endomembrane system (ES). The existence of these proteins inspired a hypothesis that all nuclear-encoded, plastid-targeted proteins initially carried signal peptides and were targeted to the ancestral primary plastid via the host ES.
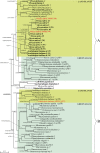
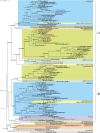
Results: We present the first phylogenetic analyses of Arabidopsis thaliana α-carbonic anhydrase (CAH1), Oryza sativa nucleotide pyrophosphatase/phosphodiesterase (NPP1), and two O. sativa α-amylases (αAmy3, αAmy7), proteins that are directed to higher plant primary plastids via the ES. We also investigated protein disulfide isomerase (RB60) from the green alga Chlamydomonas reinhardtii because of its peculiar dual post- and co-translational targeting to both the plastid and ES. Our analyses show that these proteins all are of eukaryotic rather than cyanobacterial origin, and that their non-plastid homologs are equipped with signal peptides responsible for co-translational import into the host ES. Our results indicate that vesicular trafficking of proteins to primary plastids evolved long after the cyanobacterial endosymbiosis (possibly only in higher plants) to permit their glycosylation and/or transport to more than one cellular compartment.
Conclusions: The proteins we analyzed are not relics of ES-mediated protein targeting to the ancestral primary plastid. Available data indicate that Toc- and Tic-based translocation dominated protein import into primary plastids from the beginning. Only a handful of host proteins, which already were targeted through the ES, later were adapted to reach the plastid via the vesicular trafficking. They represent a derived class of higher plant plastid-targeted proteins with an unusual evolutionary history.
Figures


Similar articles
-
Early steps in plastid evolution: current ideas and controversies.Bioessays. 2009 Nov;31(11):1219-32. doi: 10.1002/bies.200900073. Bioessays. 2009. PMID: 19847819
-
Host origin of plastid solute transporters in the first photosynthetic eukaryotes.Genome Biol. 2007;8(10):R212. doi: 10.1186/gb-2007-8-10-r212. Genome Biol. 2007. PMID: 17919328 Free PMC article.
-
Did trypanosomatid parasites contain a eukaryotic alga-derived plastid in their evolutionary past?J Parasitol. 2010 Apr;96(2):465-75. doi: 10.1645/GE-1810.1. J Parasitol. 2010. PMID: 20540605
-
On the origin of chloroplasts, import mechanisms of chloroplast-targeted proteins, and loss of photosynthetic ability - review.Folia Microbiol (Praha). 2009;54(4):303-21. doi: 10.1007/s12223-009-0048-z. Epub 2009 Oct 14. Folia Microbiol (Praha). 2009. PMID: 19826918 Review.
-
A new scenario of plastid evolution: plastid primary endosymbiosis before the divergence of the "Plantae," emended.J Plant Res. 2005 Aug;118(4):247-55. doi: 10.1007/s10265-005-0219-1. Epub 2005 Jul 20. J Plant Res. 2005. PMID: 16032387 Review.
Cited by
-
Understanding protein import in diverse non-green plastids.Front Genet. 2023 Mar 16;14:969931. doi: 10.3389/fgene.2023.969931. eCollection 2023. Front Genet. 2023. PMID: 37007964 Free PMC article. Review.
-
Protein targeting and transport as a necessary consequence of increased cellular complexity.Cold Spring Harb Perspect Biol. 2014 Aug 1;6(8):a016055. doi: 10.1101/cshperspect.a016055. Cold Spring Harb Perspect Biol. 2014. PMID: 25085907 Free PMC article. Review.
-
How Did Thylakoids Emerge in Cyanobacteria, and How Were the Primary Chloroplast and Chromatophore Acquired?Methods Mol Biol. 2024;2776:3-20. doi: 10.1007/978-1-0716-3726-5_1. Methods Mol Biol. 2024. PMID: 38502495
-
The Photosynthetic Adventure of Paulinella Spp.Results Probl Cell Differ. 2020;69:353-386. doi: 10.1007/978-3-030-51849-3_13. Results Probl Cell Differ. 2020. PMID: 33263879
-
Border control: selectivity of chloroplast protein import and regulation at the TOC-complex.Front Plant Sci. 2014 Sep 17;5:483. doi: 10.3389/fpls.2014.00483. eCollection 2014. Front Plant Sci. 2014. PMID: 25278954 Free PMC article. Review.
References
-
- Palmer JD. The symbiotic birth and spread of plastids: how many times and whodunit? J Phycol. 2003;39:4–12.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

