From bench to bedside: preclinical evaluation of a self-inactivating gammaretroviral vector for the gene therapy of X-linked chronic granulomatous disease
- PMID: 23845071
- PMCID: PMC6461155
- DOI: 10.1089/humc.2013.019
From bench to bedside: preclinical evaluation of a self-inactivating gammaretroviral vector for the gene therapy of X-linked chronic granulomatous disease
Abstract
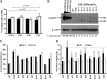
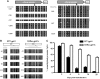
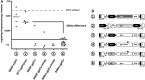
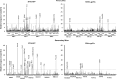
Chronic granulomatous disease (CGD) is a primary immunodeficiency characterized by impaired antimicrobial activity in phagocytic cells. As a monogenic disease affecting the hematopoietic system, CGD is amenable to gene therapy. Indeed in a phase I/II clinical trial, we demonstrated a transient resolution of bacterial and fungal infections. However, the therapeutic benefit was compromised by the occurrence of clonal dominance and malignant transformation demanding alternative vectors with equal efficacy but safety-improved features. In this work we have developed and tested a self-inactivating (SIN) gammaretroviral vector (SINfes.gp91s) containing a codon-optimized transgene (gp91(phox)) under the transcriptional control of a myeloid promoter for the gene therapy of the X-linked form of CGD (X-CGD). Gene-corrected cells protected X-CGD mice from Aspergillus fumigatus challenge at low vector copy numbers. Moreover, the SINfes.gp91s vector generates substantial amounts of superoxide in human cells transplanted into immunodeficient mice. In vitro genotoxicity assays and longitudinal high-throughput integration site analysis in transplanted mice comprising primary and secondary animals for 11 months revealed a safe integration site profile with no signs of clonal dominance.
Figures


Similar articles
-
Biochemical correction of X-CGD by a novel chimeric promoter regulating high levels of transgene expression in myeloid cells.Mol Ther. 2011 Jan;19(1):122-32. doi: 10.1038/mt.2010.226. Epub 2010 Oct 26. Mol Ther. 2011. PMID: 20978475 Free PMC article.
-
Retroviral-mediated gene transfer of gp91phox into bone marrow cells rescues defect in host defense against Aspergillus fumigatus in murine X-linked chronic granulomatous disease.Blood. 1997 Jan 1;89(1):41-8. Blood. 1997. PMID: 8978275
-
Human miR223 promoter as a novel myelo-specific promoter for chronic granulomatous disease gene therapy.Hum Gene Ther Methods. 2013 Jun;24(3):151-9. doi: 10.1089/hgtb.2012.157. Epub 2013 May 2. Hum Gene Ther Methods. 2013. PMID: 23489116 Free PMC article.
-
Chronic granulomatous disease: Clinical, molecular, and therapeutic aspects.Pediatr Allergy Immunol. 2016 May;27(3):242-53. doi: 10.1111/pai.12527. Epub 2016 Jan 21. Pediatr Allergy Immunol. 2016. PMID: 26680691 Review.
-
[Two breakthroughs in CGD studies].Nihon Rinsho Meneki Gakkai Kaishi. 2007 Feb;30(1):1-10. doi: 10.2177/jsci.30.1. Nihon Rinsho Meneki Gakkai Kaishi. 2007. PMID: 17332699 Review. Japanese.
Cited by
-
Construction of Ang2-siRNA chitosan magnetic nanoparticles and the effect on Ang2 gene expression in human malignant melanoma cells.Oncol Lett. 2016 Jun;11(6):3992-3998. doi: 10.3892/ol.2016.4539. Epub 2016 May 6. Oncol Lett. 2016. PMID: 27313729 Free PMC article.
-
Periodontal and other oral manifestations of immunodeficiency diseases.Oral Dis. 2017 Oct;23(7):866-888. doi: 10.1111/odi.12584. Epub 2016 Oct 10. Oral Dis. 2017. PMID: 27630012 Free PMC article. Review.
-
Evaluating the state of the science for adeno-associated virus integration: An integrated perspective.Mol Ther. 2022 Aug 3;30(8):2646-2663. doi: 10.1016/j.ymthe.2022.06.004. Epub 2022 Jun 10. Mol Ther. 2022. PMID: 35690906 Free PMC article. Review.
-
A Graph Based Framework to Model Virus Integration Sites.Comput Struct Biotechnol J. 2015 Nov 30;14:69-77. doi: 10.1016/j.csbj.2015.10.006. eCollection 2016. Comput Struct Biotechnol J. 2015. PMID: 27257470 Free PMC article.
-
An RNA-targeted therapy for dystrophic epidermolysis bullosa.Nucleic Acids Res. 2017 Sep 29;45(17):10259-10269. doi: 10.1093/nar/gkx669. Nucleic Acids Res. 2017. PMID: 28973459 Free PMC article.
References
-
- Aiuti A. Bacchetta R. Seger R., et al. Gene therapy for primary immunodeficiencies: part 2. Curr. Opin. Immunol. 2012;24:585–591. - PubMed
-
- Avedillo Diez I. Zychlinski D. Coci E.G., et al. Development of novel efficient SIN vectors with improved safety features for Wiskott-Aldrich Syndrome stem cell based gene therapy. Mol. Pharmaceutics. 2011;8:1525–1537. - PubMed
-
- Baum C. Von Kalle C. Staal F.J., et al. Chance or necessity? Insertional mutagenesis in gene therapy and its consequences. Mol. Ther. 2004;9:5–13. - PubMed
-
- Baum C. Modlich U. Gohring G., et al. Concise review: managing genotoxicity in the therapeutic modification of stem cells. Stem Cells. 2011;29:1479–1484. - PubMed
-
- Biffi A. Bartolomae C.C. Cesana D., et al. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood. 2011;117:5332–5339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

