Activation state-selective kinase inhibitor assay based on ion mobility-mass spectrometry
- PMID: 23845095
- PMCID: PMC3784979
- DOI: 10.1021/ac4012655
Activation state-selective kinase inhibitor assay based on ion mobility-mass spectrometry
Abstract
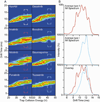
The discovery of activation state dependent kinase inhibitors, which bind specifically to the inactive conformation of the protein, is considered to be a promising pathway to improved cancer treatments. Identifying such inhibitors is challenging, however, because they can have Kd values similar to molecules known to inhibit kinase function by interacting with the active form. Further, while inhibitor induced changes within the kinase tertiary structure are significant, few technologies are able to correctly assign inhibitor binding modes in a high-throughput fashion based exclusively on protein-inhibitor complex formation and changes in local protein structure. We have developed a new assay, using ion mobility-mass spectrometry, capable of both rapidly detecting inhibitor binding and classifying the resultant kinase binding modes. Here, we demonstrate the ability of our approach to classify a broad set of kinase inhibitors, using micrograms of protein, without the need for protein modification or tagging.
Figures
Similar articles
-
Binding or bending: distinction of allosteric Abl kinase agonists from antagonists by an NMR-based conformational assay.J Am Chem Soc. 2010 May 26;132(20):7043-8. doi: 10.1021/ja101837n. J Am Chem Soc. 2010. PMID: 20450175
-
Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry.Chem Biol. 2010 Nov 24;17(11):1241-9. doi: 10.1016/j.chembiol.2010.09.010. Chem Biol. 2010. PMID: 21095574
-
Abl inhibitor BMS354825 binding mode in Abelson kinase revealed by molecular docking studies.Leukemia. 2005 Jul;19(7):1267-9. doi: 10.1038/sj.leu.2403775. Leukemia. 2005. PMID: 15858616 No abstract available.
-
New resistance mechanisms for small molecule kinase inhibitors of Abl kinase.Drug Discov Today Technol. 2014 Mar;11:5-10. doi: 10.1016/j.ddtec.2013.12.001. Drug Discov Today Technol. 2014. PMID: 24847647 Review.
-
Unexpected off-targets and paradoxical pathway activation by kinase inhibitors.ACS Chem Biol. 2015 Jan 16;10(1):234-45. doi: 10.1021/cb500886n. Epub 2015 Jan 2. ACS Chem Biol. 2015. PMID: 25531586 Review.
Cited by
-
Collisional unfolding of multiprotein complexes reveals cooperative stabilization upon ligand binding.Protein Sci. 2015 Aug;24(8):1272-81. doi: 10.1002/pro.2699. Epub 2015 May 27. Protein Sci. 2015. PMID: 25970849 Free PMC article.
-
Exploring the Conformational Landscape and Stability of Aurora A Using Ion-Mobility Mass Spectrometry and Molecular Modeling.J Am Soc Mass Spectrom. 2022 Mar 2;33(3):420-435. doi: 10.1021/jasms.1c00271. Epub 2022 Jan 31. J Am Soc Mass Spectrom. 2022. PMID: 35099954 Free PMC article.
-
Bivalent Inhibitors of c-Src Tyrosine Kinase That Bind a Regulatory Domain.Bioconjug Chem. 2016 Jul 20;27(7):1745-9. doi: 10.1021/acs.bioconjchem.6b00243. Epub 2016 Jun 22. Bioconjug Chem. 2016. PMID: 27266260 Free PMC article.
-
Native Mass Spectrometry, Ion mobility, and Collision-Induced Unfolding Categorize Malaria Antigen/Antibody Binding.J Am Soc Mass Spectrom. 2017 Nov;28(11):2515-2518. doi: 10.1007/s13361-017-1782-0. Epub 2017 Sep 5. J Am Soc Mass Spectrom. 2017. PMID: 28875466 Free PMC article.
-
A Label-free Sirtuin 1 Assay based on Droplet-Electrospray Ionization Mass Spectrometry.Anal Methods. 2016 May 7;8(17):3458-3465. doi: 10.1039/C6AY00698A. Epub 2016 Apr 7. Anal Methods. 2016. PMID: 27482292 Free PMC article.
References
-
- Krause DS, Van Etten RA. N. Engl. J. Med. 2005;353:172–187. - PubMed
-
- Deininger MWN, Goldman JM, Melo JV. Blood. 2000;96:3343–3356. - PubMed
-
- Liu Y, Gray NS. Nat. Chem. Biol. 2006;2:358–364. - PubMed
-
- Thaimattam R, Banerjee R, Miglani R, Iqbal J. Curr. Pharm. Design. 2007;13:2751–2765. - PubMed
-
- Bixby D, Talpaz M. ASH Education Program Book. 2009;2009:461–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

