Coumestrol from the national cancer Institute's natural product library is a novel inhibitor of protein kinase CK2
- PMID: 23845105
- PMCID: PMC3726451
- DOI: 10.1186/2050-6511-14-36
Coumestrol from the national cancer Institute's natural product library is a novel inhibitor of protein kinase CK2
Abstract
Background: Casein kinase 2 (CK2) is involved in various cellular events such as proliferation, apoptosis, and the cell cycle. CK2 overexpression is associated with multiple human cancers and may therefore be a promising target for cancer therapy. To identity novel classes of inhibitors for CK2, we screened a natural product library obtained from National Cancer Institute.
Methods: The quantitative luminescent kinase assay ADP-Glo™ was used to screen CK2 inhibitors from the natural product library. The same assay was used to determine cell-free dose-dependent response of CK2 inhibitors and conduct a kinetic study. Docking was performed to predict the binding patterns of selected CK2 inhibitors. Western blot analysis was used to evaluate Akt phosphorylation specific to CK2 and apoptosis effect. The cell viability assay CellTiter-Glo(®) was used to evaluate the inhibition effects of CK2 inhibitors on cancer cells.
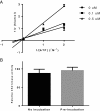
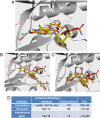
Results: We identified coumestrol as a novel reversible ATP competitive CK2 inhibitor with an IC(50) value of 228 nM. Coumestrol is a plant-derived compound that belongs to the class of phytoestrogens, natural compounds that mimic the biological activity of estrogens. In our study, coumestrol showed high selectivity among 13 kinases. The hydrogen bonds formed between coumestrol and the amino acids in the ATP binding site were first reviewed by a molecular docking study that suggested a possible interaction of coumestrol with the hinge region of ATP site of CK2. In addition, coumestrol inhibited cancer cell growth partially through down-regulation of CK2-specific Akt phosphorylation. Finally, coumestrol exerted strong inhibition effects on the growth of three cancer cell lines.
Conclusion: Our study shows that coumestrol, a novel ATP competitive and cell permeable CK2 inhibitor with submicromolar IC(50), had inhibition effects on the growth of three cancer cell lines and may represent a promising class of CK2 inhibitors.
Figures


Similar articles
-
Identification of hematein as a novel inhibitor of protein kinase CK2 from a natural product library.BMC Cancer. 2009 May 6;9:135. doi: 10.1186/1471-2407-9-135. BMC Cancer. 2009. PMID: 19419583 Free PMC article.
-
TF--a novel cell-permeable and selective inhibitor of human protein kinase CK2 induces apoptosis in the prostate cancer cell line LNCaP.Biochim Biophys Acta. 2012 Jul;1820(7):970-7. doi: 10.1016/j.bbagen.2012.02.009. Epub 2012 Feb 24. Biochim Biophys Acta. 2012. PMID: 22387500
-
In Silico and In Vitro Studies of Natural Compounds as Human CK2 Inhibitors.Curr Comput Aided Drug Des. 2021;17(2):323-331. doi: 10.2174/1573409916666200311150744. Curr Comput Aided Drug Des. 2021. PMID: 32160849
-
Small molecule modulators targeting protein kinase CK1 and CK2.Eur J Med Chem. 2019 Nov 1;181:111581. doi: 10.1016/j.ejmech.2019.111581. Epub 2019 Aug 1. Eur J Med Chem. 2019. PMID: 31400711 Review.
-
Protein kinase CK2 in health and disease: Structural bases of protein kinase CK2 inhibition.Cell Mol Life Sci. 2009 Jun;66(11-12):1868-89. doi: 10.1007/s00018-009-9155-x. Cell Mol Life Sci. 2009. PMID: 19387547 Free PMC article. Review.
Cited by
-
Compounds from Natural Sources as Protein Kinase Inhibitors.Biomolecules. 2020 Nov 12;10(11):1546. doi: 10.3390/biom10111546. Biomolecules. 2020. PMID: 33198400 Free PMC article. Review.
-
New Dual CK2/HDAC1 Inhibitors with Nanomolar Inhibitory Activity against Both Enzymes.ACS Med Chem Lett. 2020 Apr 7;11(5):713-719. doi: 10.1021/acsmedchemlett.9b00561. eCollection 2020 May 14. ACS Med Chem Lett. 2020. PMID: 32435375 Free PMC article.
-
Pterocarpan-enriched soy leaf extract ameliorates insulin sensitivity and pancreatic β-cell proliferation in type 2 diabetic mice.Molecules. 2014 Nov 13;19(11):18493-510. doi: 10.3390/molecules191118493. Molecules. 2014. PMID: 25401395 Free PMC article.
-
The daidzein metabolite, 6,7,4'-Trihydroxyisoflavone, is a novel inhibitor of PKCα in suppressing solar UV-induced matrix metalloproteinase 1.Int J Mol Sci. 2014 Nov 19;15(11):21419-32. doi: 10.3390/ijms151121419. Int J Mol Sci. 2014. PMID: 25415304 Free PMC article.
-
Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance.Antibiotics (Basel). 2020 Aug 4;9(8):480. doi: 10.3390/antibiotics9080480. Antibiotics (Basel). 2020. PMID: 32759771 Free PMC article. Review.
References
-
- Ghavidel A, Hockman DJ, Schultz MC. A review of progress towards elucidating the role of protein kinase CK2 in polymerase III transcription: regulation of the TATA binding protein. Mol Cell Biochem. 1999;191(1–2):143–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

