Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome
- PMID: 23845947
- PMCID: PMC4375961
- DOI: 10.1126/science.1233151
Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome
Abstract
Wiskott-Aldrich syndrome (WAS) is an inherited immunodeficiency caused by mutations in the gene encoding WASP, a protein regulating the cytoskeleton. Hematopoietic stem/progenitor cell (HSPC) transplants can be curative, but, when matched donors are unavailable, infusion of autologous HSPCs modified ex vivo by gene therapy is an alternative approach. We used a lentiviral vector encoding functional WASP to genetically correct HSPCs from three WAS patients and reinfused the cells after a reduced-intensity conditioning regimen. All three patients showed stable engraftment of WASP-expressing cells and improvements in platelet counts, immune functions, and clinical scores. Vector integration analyses revealed highly polyclonal and multilineage haematopoiesis resulting from the gene-corrected HSPCs. Lentiviral gene therapy did not induce selection of integrations near oncogenes, and no aberrant clonal expansion was observed after 20 to 32 months. Although extended clinical observation is required to establish long-term safety, lentiviral gene therapy represents a promising treatment for WAS.
Trial registration: ClinicalTrials.gov NCT01515462.
Figures
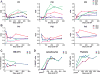
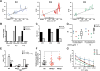
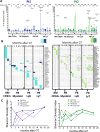
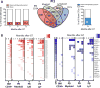

Comment in
-
Gene therapy: primed for take-off.Nature. 2013 Aug 15;500(7462):280-2. doi: 10.1038/500280a. Nature. 2013. PMID: 23955226 No abstract available.
-
Medicine. Gene therapy that works.Science. 2013 Aug 23;341(6148):853-5. doi: 10.1126/science.1242551. Science. 2013. PMID: 23970689 No abstract available.
-
Broadening the indications for hematopoietic stem cell genetic therapies.Cell Stem Cell. 2013 Sep 5;13(3):263-4. doi: 10.1016/j.stem.2013.08.006. Cell Stem Cell. 2013. PMID: 24012366
-
A possible turning point in the hematopoietic stem cell gene therapy for primary immunodeficiency diseases? Lentiviral vectors could take the place of retroviral vectors.Expert Rev Clin Immunol. 2013 Nov;9(11):1015-8. doi: 10.1586/1744666X.2013.850416. Expert Rev Clin Immunol. 2013. PMID: 24168409
References
-
- Notarangelo LD, Miao CH, Ochs HD. Wiskott-Aldrich syndrome. Curr Opin Hematol. 2008;15:30. - PubMed
-
- Dupre L, et al. Lentiviral vector-mediated gene transfer in T cells from Wiskott-Aldrich syndrome patients leads to functional correction. Mol Ther. 2004;10:903. - PubMed
-
- Bosticardo M, Marangoni F, Aiuti A, Villa A, Roncarolo MG. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood. 2009;113:6288. - PubMed
-
- Ochs HD, Filipovich AH, Veys P, Cowan MJ, K N. Wiskott-Aldrich syndrome: diagnosis, clinical and laboratory manifestations, and treatment. Biol Blood Marrow Transplant. 2009;15:84. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

