Closed-loop optogenetic intervention in mice
- PMID: 23845961
- PMCID: PMC3988315
- DOI: 10.1038/nprot.2013.080
Closed-loop optogenetic intervention in mice
Abstract
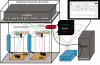
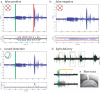
Optogenetic interventions offer novel ways of probing, in a temporally specific manner, the roles of specific cell types in neuronal network functions of awake, behaving animals. Despite the unique potential for temporally specific optogenetic intervention in disease states, a major hurdle in its broad application to unpredictable brain states in a laboratory setting is constructing a real-time responsive system. We recently created a closed-loop system for stopping spontaneous seizures in chronically epileptic mice by using optogenetic intervention. This system performs with a very high sensitivity and specificity, and the strategy is not only relevant to epilepsy but also can also be used to react to diverse brain states in real time, with optogenetic or other interventions. The protocol presented here is highly modular and requires variable amounts of time to perform. We describe the basic construction of a complete system, and we include our downloadable custom closed-loop detection software, which can be used for this purpose.
Figures




References
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

