Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial
- PMID: 23846316
- PMCID: PMC3787916
- DOI: 10.1378/chest.13-0178
Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial
Abstract
Background: The combination of fluticasone furoate (FF), a novel inhaled corticosteroid (ICS), and vilanterol (VI), a long-acting β2 agonist, is under development as a once-daily treatment of asthma and COPD. The aim of this study was to compare the efficacy of FF/VI with fluticasone propionate (FP)/salmeterol (SAL) in patients with persistent asthma uncontrolled on a medium dose of ICS.
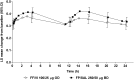
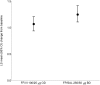
Methods: In a randomized, double-blind, double-dummy, parallel group study, 806 patients received FF/VI (100/25 μg, n = 403) once daily in the evening delivered through ELLIPTA (GlaxoSmithKline) dry powder inhaler, or FP/SAL (250/50 μg, n = 403) bid through DISKUS/ACCUHALER (GlaxoSmithKline). The primary efficacy measure was 0- to 24-h serial weighted mean (wm) FEV1 after 24 weeks of treatment.
Results: Improvements from baseline in 0- to 24-h wmFEV1 were observed with both FF/VI (341 mL) and FP/SAL (377 mL); the adjusted mean treatment difference was not statistically significant (-37 mL; 95% CI, -88 to 15, P = 0.162). There were no differences between 0- to 4-h serial wmFEV1, trough FEV1, and asthma control and quality-of-life questionnaire scores. There was no difference in reported exacerbations between treatments. Both treatments were well tolerated, with no clinically relevant effect on urinary cortisol excretion or vital signs and no treatment-related serious adverse events.
Conclusions: The efficacy of once-daily FF/VI was similar to bid FP/SAL in improving lung function in patients with persistent asthma. No safety issues were identified.
Trial registration: ClinicalTrials.gov NCT01147848.
Figures


References
-
- National Institutes of Health Guidelines for the diagnosis and management of asthma (EPR-3) 2007. NIH publication 08-4051. National Heart, Lung, and Blood Institute website. http://www.nhlbi.nih.gov/guidelines/asthma/index.htm. Accessed July 12, 2013
-
- Global Initiative for Asthma Global strategy for asthma management and prevention. Global Initiative for Asthma website. http://www.ginasthma.org/guidelines-gina-report-global-strategy-for-asth.... Updated 2012. Accessed July 12, 2013
-
- Greenstone IR, Ni Chroinin MN, Masse V, et al. Combination of inhaled long-acting beta2-agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database Syst Rev. 2005;(4):CD005533. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

