Contributions of leukocyte angiotensin-converting enzyme to development of atherosclerosis
- PMID: 23846498
- PMCID: PMC3868562
- DOI: 10.1161/ATVBAHA.113.301777
Contributions of leukocyte angiotensin-converting enzyme to development of atherosclerosis
Abstract
Objective: This study determined the role of angiotensin-converting enzyme (ACE) on the development of angiotensin I-induced atherosclerosis and the contribution of leukocyte-specific expression of this enzyme.
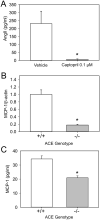
Approach and results: To define the contribution of ACE-dependent activity to angiotensin II synthesis in atherosclerotic development, male low-density lipoprotein receptor(-/-) mice were fed a fat-enriched diet and infused with either angiotensin I or angiotensin II. The same infusion rate of these peptides had equivalent effects on atherosclerotic development. Coinfusion of an ACE inhibitor, enalapril, ablated angiotensin I-augmented atherosclerosis but had no effect on angiotensin II-induced lesion development. ACE protein was detected in several cell types in atherosclerotic lesions, with a predominance in macrophages. This cell type secreted angiotensin II, which was ablated by ACE inhibition. To study whether leukocyte ACE contributed to atherosclerosis, irradiated male low-density lipoprotein receptor(-/-) mice were repopulated with bone marrow-derived cells from either ACE(+/+) or ACE(-/-) mice and fed the fat-enriched diet for 12 weeks. Chimeric mice with ACE deficiency in bone marrow-derived cells had modestly reduced atherosclerotic lesions in aortic arches but had no effects in aortic roots.
Conclusions: ACE mediates angiotensin I-induced atherosclerosis, and ACE expression in leukocytes modestly contributes to atherosclerotic development in hypercholesterolemic mice.
Keywords: angiotensin-converting enzyme inhibitors; angiotensins; atherosclerosis; hypercholesterolemia; macrophages; renin.
Conflict of interest statement
Figures



Similar articles
-
Angiotensin-Converting Enzyme in Smooth Muscle Cells Promotes Atherosclerosis-Brief Report.Arterioscler Thromb Vasc Biol. 2016 Jun;36(6):1085-9. doi: 10.1161/ATVBAHA.115.307038. Epub 2016 Apr 7. Arterioscler Thromb Vasc Biol. 2016. PMID: 27055902 Free PMC article.
-
Angiotensin-converting enzyme 2 deficiency in whole body or bone marrow-derived cells increases atherosclerosis in low-density lipoprotein receptor-/- mice.Arterioscler Thromb Vasc Biol. 2011 Apr;31(4):758-65. doi: 10.1161/ATVBAHA.110.221614. Epub 2011 Jan 20. Arterioscler Thromb Vasc Biol. 2011. PMID: 21252069 Free PMC article.
-
Leukocyte Calpain Deficiency Reduces Angiotensin II-Induced Inflammation and Atherosclerosis But Not Abdominal Aortic Aneurysms in Mice.Arterioscler Thromb Vasc Biol. 2016 May;36(5):835-45. doi: 10.1161/ATVBAHA.116.307285. Epub 2016 Mar 10. Arterioscler Thromb Vasc Biol. 2016. PMID: 26966280 Free PMC article.
-
ACE-inhibitors and atherosclerosis.Eur J Epidemiol. 1992 May;8 Suppl 1:129-33. doi: 10.1007/BF00145364. Eur J Epidemiol. 1992. PMID: 1324185 Review.
-
Classical and nonclassical effects of angiotensin-converting enzyme: How increased ACE enhances myeloid immune function.J Biol Chem. 2024 Jun;300(6):107388. doi: 10.1016/j.jbc.2024.107388. Epub 2024 May 17. J Biol Chem. 2024. PMID: 38763333 Free PMC article. Review.
Cited by
-
Angiotensinogen Exerts Effects Independent of Angiotensin II.Arterioscler Thromb Vasc Biol. 2016 Feb;36(2):256-65. doi: 10.1161/ATVBAHA.115.306740. Epub 2015 Dec 17. Arterioscler Thromb Vasc Biol. 2016. PMID: 26681751 Free PMC article.
-
Significant Association of Interleukin-16 Genetic Variations to Taiwanese Lung Cancer.In Vivo. 2020 May-Jun;34(3):1117-1123. doi: 10.21873/invivo.11883. In Vivo. 2020. PMID: 32354900 Free PMC article.
-
Angiotensin-Converting Enzyme in Smooth Muscle Cells Promotes Atherosclerosis-Brief Report.Arterioscler Thromb Vasc Biol. 2016 Jun;36(6):1085-9. doi: 10.1161/ATVBAHA.115.307038. Epub 2016 Apr 7. Arterioscler Thromb Vasc Biol. 2016. PMID: 27055902 Free PMC article.
-
Uremic conditions drive human monocytes to pro-atherogenic differentiation via an angiotensin-dependent mechanism.PLoS One. 2014 Jul 8;9(7):e102137. doi: 10.1371/journal.pone.0102137. eCollection 2014. PLoS One. 2014. PMID: 25003524 Free PMC article.
-
Noncanonical Mechanisms for Direct Bone Marrow Generating Ang II (Angiotensin II) Predominate in CD68 Positive Myeloid Lineage Cells.Hypertension. 2020 Feb;75(2):500-509. doi: 10.1161/HYPERTENSIONAHA.119.13754. Epub 2019 Dec 9. Hypertension. 2020. PMID: 31813348 Free PMC article.
References
-
- Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. - PubMed
-
- Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor -/- mice. Ann NY Acad Sci. 1999;892:108–118. - PubMed
-
- Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

