Individual interactions of the b subunits within the stator of the Escherichia coli ATP synthase
- PMID: 23846684
- PMCID: PMC3750146
- DOI: 10.1074/jbc.M113.465633
Individual interactions of the b subunits within the stator of the Escherichia coli ATP synthase
Abstract
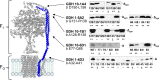
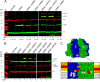
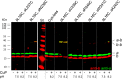
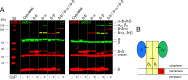
FOF1 ATP synthases are rotary nanomotors that couple proton translocation across biological membranes to the synthesis/hydrolysis of ATP. During catalysis, the peripheral stalk, composed of two b subunits and subunit δ in Escherichia coli, counteracts the torque generated by the rotation of the central stalk. Here we characterize individual interactions of the b subunits within the stator by use of monoclonal antibodies and nearest neighbor analyses via intersubunit disulfide bond formation. Antibody binding studies revealed that the C-terminal region of one of the two b subunits is principally involved in the binding of subunit δ, whereas the other one is accessible to antibody binding without impact on the function of FOF1. Individually substituted cysteine pairs suitable for disulfide cross-linking between the b subunits and the other stator subunits (b-α, b-β, b-δ, and b-a) were screened and combined with each other to discriminate between the two b subunits (i.e. bI and bII). The results show the b dimer to be located at a non-catalytic α/β cleft, with bI close to subunit α, whereas bII is proximal to subunit β. Furthermore, bI can be linked to subunit δ as well as to subunit a. Among the subcomplexes formed were a-bI-α, bII-β, α-bI-bII-β, and a-bI-δ. Taken together, the data obtained define the different positions of the two b subunits at a non-catalytic interface and imply that each b subunit has a different role in generating stability within the stator. We suggest that bI is functionally related to the single b subunit present in mitochondrial ATP synthase.
Keywords: ATP Synthase; Disulfide; Escherichia coli; F-ATPase; Membrane Proteins; Peripheral Stator Stalk; Protein Cross-linking.
Figures




Similar articles
-
Subunit δ is the key player for assembly of the H(+)-translocating unit of Escherichia coli F(O)F1 ATP synthase.J Biol Chem. 2013 Sep 6;288(36):25880-25894. doi: 10.1074/jbc.M113.484675. Epub 2013 Jul 17. J Biol Chem. 2013. PMID: 23864656 Free PMC article.
-
Rotor/Stator interactions of the epsilon subunit in Escherichia coli ATP synthase and implications for enzyme regulation.J Biol Chem. 2004 Aug 20;279(34):35616-21. doi: 10.1074/jbc.M405012200. Epub 2004 Jun 15. J Biol Chem. 2004. PMID: 15199054
-
Assembly of the stator in Escherichia coli ATP synthase. Complexation of alpha subunit with other F1 subunits is prerequisite for delta subunit binding to the N-terminal region of alpha.Biochemistry. 2006 Dec 26;45(51):15893-902. doi: 10.1021/bi0619730. Epub 2006 Dec 5. Biochemistry. 2006. PMID: 17176112 Free PMC article.
-
ATP synthase from Escherichia coli: Mechanism of rotational catalysis, and inhibition with the ε subunit and phytopolyphenols.Biochim Biophys Acta. 2016 Feb;1857(2):129-140. doi: 10.1016/j.bbabio.2015.11.005. Epub 2015 Nov 14. Biochim Biophys Acta. 2016. PMID: 26589785 Review.
-
The regulatory subunit ε in Escherichia coli FOF1-ATP synthase.Biochim Biophys Acta Bioenerg. 2018 Sep;1859(9):775-788. doi: 10.1016/j.bbabio.2018.06.013. Epub 2018 Jun 20. Biochim Biophys Acta Bioenerg. 2018. PMID: 29932911 Free PMC article. Review.
Cited by
-
Subunit δ is the key player for assembly of the H(+)-translocating unit of Escherichia coli F(O)F1 ATP synthase.J Biol Chem. 2013 Sep 6;288(36):25880-25894. doi: 10.1074/jbc.M113.484675. Epub 2013 Jul 17. J Biol Chem. 2013. PMID: 23864656 Free PMC article.
-
Structural Asymmetry and Kinetic Limping of Single Rotary F-ATP Synthases.Molecules. 2019 Jan 30;24(3):504. doi: 10.3390/molecules24030504. Molecules. 2019. PMID: 30704145 Free PMC article. Review.
-
Escherichia coli F1Fo-ATP synthase with a b/δ fusion protein allows analysis of the function of the individual b subunits.J Biol Chem. 2013 Sep 13;288(37):26441-7. doi: 10.1074/jbc.M113.503722. Epub 2013 Jul 26. J Biol Chem. 2013. PMID: 23893411 Free PMC article.
-
Low membrane fluidity triggers lipid phase separation and protein segregation in living bacteria.EMBO J. 2022 Mar 1;41(5):e109800. doi: 10.15252/embj.2021109800. Epub 2022 Jan 17. EMBO J. 2022. PMID: 35037270 Free PMC article.
-
The Efficiency of Lemon Essential Oil-Based Nanoemulsions on the Inhibition of Phomopsis sp. and Reduction of Postharvest Decay of Kiwifruit.Foods. 2022 May 22;11(10):1510. doi: 10.3390/foods11101510. Foods. 2022. PMID: 35627080 Free PMC article.
References
-
- Junge W., Sielaff H., Engelbrecht S. (2009) Torque generation and elastic power transmission in the rotary FOF1-ATPase. Nature 459, 364–370 - PubMed
-
- von Ballmoos C., Wiedenmann A., Dimroth P. (2009) Essentials for ATP synthesis by F1FO ATP synthases. Annu. Rev. Biochem. 78, 649–672 - PubMed
-
- von Ballmoos C., Cook G. M., Dimroth P. (2008) Unique rotor ATP synthase and its biological diversity. Annu. Rev. Biophys. 37, 43–64 - PubMed
-
- Cross R. L., Müller V. (2004) The evolution of A-, F-, and V-type ATP synthases and ATPases. Reversals in function and changes in the H+/ATP coupling ratio. FEBS Lett. 576, 1–4 - PubMed
-
- Muench S. P., Trinick J., Harrison M. A. (2011) Structural divergence of the rotary ATPases. Q. Rev. Biophys. 44, 311–356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

