Endocytosis of Mycobacterium tuberculosis heat shock protein 60 is required to induce interleukin-10 production in macrophages
- PMID: 23846686
- PMCID: PMC3750191
- DOI: 10.1074/jbc.M113.461004
Endocytosis of Mycobacterium tuberculosis heat shock protein 60 is required to induce interleukin-10 production in macrophages
Abstract
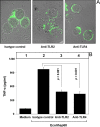
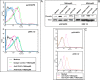
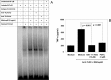
Understanding the signaling pathways involved in the regulation of anti-inflammatory and pro-inflammatory responses in tuberculosis is extremely important in tailoring a macrophage innate response to promote anti-tuberculosis immunity in the host. Although the role of toll-like receptors (TLRs) in the regulation of anti-inflammatory and pro-inflammatory responses is known, the detailed molecular mechanisms by which the Mycobacterium tuberculosis bacteria modulate these innate responses are not clearly understood. In this study, we demonstrate that M. tuberculosis heat shock protein 60 (Mtbhsp60, Cpn60.1, and Rv3417c) interacts with both TLR2 and TLR4 receptors, but its interaction with TLR2 leads to clathrin-dependent endocytosis resulting in an increased production of interleukin (IL)-10 and activated p38 MAPK. Blockage of TLR2-mediated endocytosis inhibited IL-10 production but induced production of tumor necrosis factor (TNF)-α and activated ERK1/2. In contrast, upon interaction with TLR4, Mtbhsp60 remained predominantly localized on the cell surface due to poorer endocytosis of the protein that led to decreased IL-10 production and p38 MAPK activation. The Escherichia coli homologue of hsp60 was found to be retained mainly on the macrophage surface upon interaction with either TLR2 or TLR4 that triggered predominantly a pro-inflammatory-type immune response. Our data suggest that cellular localization of Mtbhsp60 upon interaction with TLRs dictates the type of polarization in the innate immune responses in macrophages. This information is likely to help us in tailoring the host protective immune responses against M. tuberculosis.
Keywords: Cytokine; Endocytosis; MAP Kinases (MAPKs); Mycobacterium tuberculosis; Toll-like Receptors (TLR).
Figures




Similar articles
-
The PPE18 of Mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage.J Immunol. 2009 Nov 15;183(10):6269-81. doi: 10.4049/jimmunol.0901367. Epub 2009 Oct 30. J Immunol. 2009. PMID: 19880448
-
Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan.J Biol Chem. 2007 Sep 7;282(36):26014-25. doi: 10.1074/jbc.M702690200. Epub 2007 Jul 6. J Biol Chem. 2007. PMID: 17617634
-
Mycobacterium tuberculosis heat shock protein 60 modulates immune response to PPD by manipulating the surface expression of TLR2 on macrophages.Cell Microbiol. 2008 Aug;10(8):1711-22. doi: 10.1111/j.1462-5822.2008.01161.x. Epub 2008 Apr 17. Cell Microbiol. 2008. PMID: 18419772
-
Host-mycobacteria conflict: Immune responses of the host vs. the mycobacteria TLR2 and TLR4 ligands and concomitant host-directed therapy.Microbiol Res. 2022 Nov;264:127153. doi: 10.1016/j.micres.2022.127153. Epub 2022 Jul 30. Microbiol Res. 2022. PMID: 35994955 Review.
-
Innate immunity in tuberculosis: myths and truth.Microbes Infect. 2008 Jul;10(9):995-1004. doi: 10.1016/j.micinf.2008.07.039. Epub 2008 Aug 13. Microbes Infect. 2008. PMID: 18762264 Review.
Cited by
-
In-Vivo Gene Signatures of Mycobacterium tuberculosis in C3HeB/FeJ Mice.PLoS One. 2015 Aug 13;10(8):e0135208. doi: 10.1371/journal.pone.0135208. eCollection 2015. PLoS One. 2015. PMID: 26270051 Free PMC article.
-
Interaction of TLR4 and TLR8 in the Innate Immune Response against Mycobacterium Tuberculosis.Int J Mol Sci. 2021 Feb 4;22(4):1560. doi: 10.3390/ijms22041560. Int J Mol Sci. 2021. PMID: 33557133 Free PMC article.
-
The PPE2 protein of Mycobacterium tuberculosis translocates to host nucleus and inhibits nitric oxide production.Sci Rep. 2017 Jan 10;7:39706. doi: 10.1038/srep39706. Sci Rep. 2017. PMID: 28071726 Free PMC article.
-
TREM2 Promotes Immune Evasion by Mycobacterium tuberculosis in Human Macrophages.mBio. 2022 Aug 30;13(4):e0145622. doi: 10.1128/mbio.01456-22. Epub 2022 Aug 4. mBio. 2022. PMID: 35924849 Free PMC article.
-
Macrophage: A Cell With Many Faces and Functions in Tuberculosis.Front Immunol. 2022 May 6;13:747799. doi: 10.3389/fimmu.2022.747799. eCollection 2022. Front Immunol. 2022. PMID: 35603185 Free PMC article. Review.
References
-
- Schluger N. W., Rom W. N. (1998) The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 157, 679–691 - PubMed
-
- Rook G. A., Dheda K., Zumla A. (2005) Immune responses to tuberculosis in developing countries: implications for new vaccines. Nat. Rev. Immunol. 5, 661–667 - PubMed
-
- Flynn J. L., Goldstein M. M., Chan J., Triebold K. J., Pfeffer K., Lowenstein C. J., Schreiber R., Mak T. W., Bloom B. R. (1995) Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2, 561–572 - PubMed
-
- Flynn J. L. (2004) Immunology of tuberculosis and implications in vaccine development. Tuberculosis 84, 93–101 - PubMed
-
- Quesniaux V. F., Jacobs M., Allie N., Grivennikov S., Nedospasov S. A., Garcia I., Olleros M. L., Shebzukhov Y., Kuprash D., Vasseur V., Rose S., Court N., Vacher R., Ryffel B. (2010) TNF in host resistance to tuberculosis infection. Curr. Dir. Autoimmun. 11, 157–179 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

