Disruption of multivesicular body vesicles does not affect major histocompatibility complex (MHC) class II-peptide complex formation and antigen presentation by dendritic cells
- PMID: 23846690
- PMCID: PMC3750131
- DOI: 10.1074/jbc.M113.461996
Disruption of multivesicular body vesicles does not affect major histocompatibility complex (MHC) class II-peptide complex formation and antigen presentation by dendritic cells
Abstract
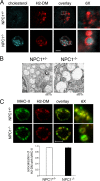
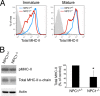
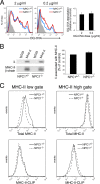
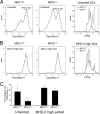
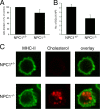
The antigen processing compartments in antigen-presenting cells (APCs) have well known characteristics of multivesicular bodies (MVBs). However, the importance of MVB integrity to APC function remains unknown. In this study, we have altered the ultrastructure of the MVB by perturbing cholesterol content genetically through the use of a deletion of the lipid transporter Niemann-Pick type C1 (NPC1). Immunofluorescence and electron microscopic analyses reveal that the antigen processing compartments in NPC1(-/-) dendritic cells (DCs) have an abnormal ultrastructure in that the organelles are enlarged and the intraluminal vesicles are almost completely absent and those remaining are completely disorganized. MHC-II is restricted to the limiting membrane of these enlarged MVBs where it colocalizes with the peptide editor H2-DM. Curiously, proteolytic removal of the chaperone protein Invariant chain from MHC-II, degradation of internalized foreign antigens, and antigenic-peptide binding to nascent MHC-II are normal in NPC1(-/-) DCs. Antigen-pulsed NPC1(-/-) DCs are able to effectively activate antigen-specific CD4 T cells in vitro, and immunization of NPC1(-/-) mice reveals surprisingly normal CD4 T cell activation in vivo. Our data thus reveal that the localization of MHC-II on the intraluminal vesicles of multivesicular antigen processing compartments is not required for efficient antigen presentation by DCs.
Keywords: Antigen Presentation; Antigen Processing; Endosomes; Major Histocompatibility Complex (MHC); Niemann-Pick Disease Type C; T Cell.
Figures
References
-
- Kleijmeer M., Ramm G., Schuurhuis D., Griffith J., Rescigno M., Ricciardi-Castagnoli P., Rudensky A. Y., Ossendorp F., Melief C. J., Stoorvogel W., Geuze H. J. (2001) Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J. Cell Biol. 155, 53–63 - PMC - PubMed
-
- Zwart W., Griekspoor A., Kuijl C., Marsman M., van Rheenen J., Janssen H., Calafat J., van Ham M., Janssen L., van Lith M., Jalink K., Neefjes J. (2005) Spatial separation of HLA-DM/HLA-DR interactions within MIIC and phagosome-induced immune escape. Immunity 22, 221–233 - PubMed
-
- Carstea E. D., Morris J. A., Coleman K. G., Loftus S. K., Zhang D., Cummings C., Gu J., Rosenfeld M. A., Pavan W. J., Krizman D. B., Nagle J., Polymeropoulos M. H., Sturley S. L., Ioannou Y. A., Higgins M. E., Comly M., Cooney A., Brown A., Kaneski C. R., Blanchette-Mackie E. J., Dwyer N. K., Neufeld E. B., Chang T. Y., Liscum L., Strauss J. F., 3rd, Ohno K., Zeigler M., Carmi R., Sokol J., Markie D., O'Neill R. R., van Diggelen O. P., Elleder M., Patterson M. C., Brady R. O., Vanier M. T., Pentchev P. G., Tagle D. A. (1997) Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 277, 228–231 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

