Structural model of the anion exchanger 1 (SLC4A1) and identification of transmembrane segments forming the transport site
- PMID: 23846695
- PMCID: PMC3772184
- DOI: 10.1074/jbc.M113.465989
Structural model of the anion exchanger 1 (SLC4A1) and identification of transmembrane segments forming the transport site
Abstract
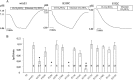
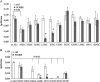
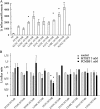
The anion exchanger 1 (AE1), a member of bicarbonate transporter family SLC4, mediates an electroneutral chloride/bicarbonate exchange in physiological conditions. However, some point mutations in AE1 membrane-spanning domain convert the electroneutral anion exchanger into a Na(+) and K(+) conductance or induce a cation leak in a still functional anion exchanger. The molecular determinants that govern ion movement through this transporter are still unknown. The present study was intended to identify the ion translocation pathway within AE1. In the absence of a resolutive three-dimensional structure of AE1 membrane-spanning domain, in silico modeling combined with site-directed mutagenesis experiments was done. A structural model of AE1 membrane-spanning domain is proposed, and this model is based on the structure of a uracil-proton symporter. This model was used to design cysteine-scanning mutagenesis on transmembrane (TM) segments 3 and 5. By measuring AE1 anion exchange activity or cation leak, it is proposed that there is a unique transport site comprising TM3-5 and TM8 that should function as an anion exchanger and a cation leak.
Keywords: Anion Exchanger 1; Anion Transport; Band 3; Erythrocyte; Homology Modeling; Ion Channels; Protein Structure; SLC4A1.
Figures



Similar articles
-
Dual transport properties of anion exchanger 1: the same transmembrane segment is involved in anion exchange and in a cation leak.J Biol Chem. 2011 Mar 18;286(11):8909-16. doi: 10.1074/jbc.M110.166819. Epub 2011 Jan 21. J Biol Chem. 2011. PMID: 21257764 Free PMC article.
-
Point mutations involved in red cell stomatocytosis convert the electroneutral anion exchanger 1 to a nonselective cation conductance.Blood. 2007 Sep 15;110(6):2158-65. doi: 10.1182/blood-2006-12-063420. Epub 2007 Jun 6. Blood. 2007. PMID: 17554061
-
Cation transport activity of anion exchanger 1 mutations found in inherited distal renal tubular acidosis.Am J Physiol Renal Physiol. 2008 Aug;295(2):F343-50. doi: 10.1152/ajprenal.00587.2007. Epub 2008 Jun 4. Am J Physiol Renal Physiol. 2008. PMID: 18524859
-
Molecular physiology of SLC4 anion exchangers.Exp Physiol. 2006 Jan;91(1):153-61. doi: 10.1113/expphysiol.2005.031765. Epub 2005 Oct 20. Exp Physiol. 2006. PMID: 16239253 Review.
-
The association between familial distal renal tubular acidosis and mutations in the red cell anion exchanger (band 3, AE1) gene.Biochem Cell Biol. 1998;76(5):723-8. doi: 10.1139/bcb-76-5-723. Biochem Cell Biol. 1998. PMID: 10353704 Review.
Cited by
-
Repository of Enriched Structures of Proteins Involved in the Red Blood Cell Environment (RESPIRE).PLoS One. 2019 Feb 22;14(2):e0211043. doi: 10.1371/journal.pone.0211043. eCollection 2019. PLoS One. 2019. PMID: 30794542 Free PMC article.
-
The Bicarbonate Transporter SLC4A7 Plays a Key Role in Macrophage Phagosome Acidification.Cell Host Microbe. 2018 Jun 13;23(6):766-774.e5. doi: 10.1016/j.chom.2018.04.013. Epub 2018 May 17. Cell Host Microbe. 2018. PMID: 29779931 Free PMC article.
-
Hereditary Spherocytosis: Can Next-Generation Sequencing of the Five Most Frequently Affected Genes Replace Time-Consuming Functional Investigations?Int J Mol Sci. 2023 Nov 30;24(23):17021. doi: 10.3390/ijms242317021. Int J Mol Sci. 2023. PMID: 38069343 Free PMC article.
-
Solute carriers keep on rockin'.Nat Struct Mol Biol. 2015 Oct;22(10):752-4. doi: 10.1038/nsmb.3104. Nat Struct Mol Biol. 2015. PMID: 26439635 No abstract available.
-
Intercalated Cell Depletion and Vacuolar H+-ATPase Mistargeting in an Ae1 R607H Knockin Model.J Am Soc Nephrol. 2017 May;28(5):1507-1520. doi: 10.1681/ASN.2016020169. Epub 2016 Dec 8. J Am Soc Nephrol. 2017. PMID: 27932475 Free PMC article.
References
-
- Bamberg E., Passow H. (eds) (1992) The Band 3 Proteins: Anion Transporters, Binding Proteins and Senescent Antigens, Elsevier, Amsterdam, Netherlands
-
- Pucéat M., Korichneva I., Cassoly R., Vassort G. (1995) Identification of band 3-like proteins and Cl−/HCO3− exchange in isolated cardiomyocytes. J. Biol. Chem. 270, 1315–1322 - PubMed
-
- Guizouarn H., Martial S., Gabillat N., Borgese F. (2007) Point mutations involved in red cell stomatocytosis convert the electroneutral anion exchanger 1 to a nonselective cation conductance. Blood 110, 2158–2165 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

