Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein
- PMID: 23846699
- PMCID: PMC3745335
- DOI: 10.1074/jbc.M113.470674
Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein
Abstract
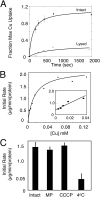
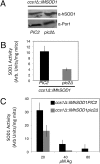
Saccharomyces cerevisiae must import copper into the mitochondrial matrix for eventual assembly of cytochrome c oxidase. This copper is bound to an anionic fluorescent molecule known as the copper ligand (CuL). Here, we identify for the first time a mitochondrial carrier family protein capable of importing copper into the matrix. In vitro transport of the CuL into the mitochondrial matrix was saturable and temperature-dependent. Strains with a deletion of PIC2 grew poorly on copper-deficient non-fermentable medium supplemented with silver and under respiratory conditions when challenged with a matrix-targeted copper competitor. Mitochondria from pic2Δ cells had lower total mitochondrial copper and exhibited a decreased capacity for copper uptake. Heterologous expression of Pic2 in Lactococcus lactis significantly enhanced CuL transport into these cells. Therefore, we propose a novel role for Pic2 in copper import into mitochondria.
Keywords: Copper; Copper Transport; Cytochrome c Oxidase; Mitochondria; Mitochondrial Carrier Family; Silver; Yeast; Yeast Metabolism.
Figures






References
-
- Cobine P. A., Pierrel F., Winge D. R. (2006) Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim. Biophys. Acta. 1763, 759–772 - PubMed
-
- Huffman D. L., O'Halloran T. V. (2001) Function, structure, and mechanism of intracellular copper trafficking proteins. Annu. Rev. Biochem. 70, 677–701 - PubMed
-
- Dancis A., Yuan D. S., Haile D., Askwith C., Eide D., Moehle C., Kaplan J., Klausner R. D. (1994) Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76, 393–402 - PubMed
-
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science 272, 1136–1144 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

