Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder
- PMID: 23846733
- PMCID: PMC3874085
- DOI: 10.1176/appi.ajp.2013.12010127
Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder
Abstract
Objective: The purpose of this study was to determine whether metformin promotes weight loss in overweight outpatients with chronic schizophrenia or schizoaffective disorder.
Method: In a double-blind study, 148 clinically stable, overweight (body mass index [BMI] ≥27) outpatients with chronic schizophrenia or schizoaffective disorder were randomly assigned to receive 16 weeks of metformin or placebo. Metformin was titrated up to 1,000 mg twice daily, as tolerated. All patients continued to receive their prestudy medications, and all received weekly diet and exercise counseling. The primary outcome measure was change in body weight from baseline to week 16.
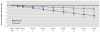
Results: Fifty-eight (77.3%) patients who received metformin and 58 (81.7%) who received placebo completed 16 weeks of treatment. Mean change in body weight was -3.0 kg (95% CI=-4.0 to -2.0) for the metformin group and -1.0 kg (95% CI=-2.0 to 0.0) for the placebo group, with a between-group difference of -2.0 kg (95% CI=-3.4 to -0.6). Metformin also demonstrated a significant between-group advantage for BMI (-0.7; 95% CI=-1.1 to -0.2), triglyceride level (-20.2 mg/dL; 95% CI=-39.2 to -1.3), and hemoglobin A1c level (-0.07%; 95% CI=-0.14 to -0.004). Metformin-associated side effects were mostly gastrointestinal and generally transient, and they rarely led to treatment discontinuation.
Conclusions: Metformin was modestly effective in reducing weight and other risk factors for cardiovascular disease in clinically stable, overweight outpatients with chronic schizophrenia or schizoaffective disorder over 16 weeks. A significant time-by-treatment interaction suggests that benefits of metformin may continue to accrue with longer treatment. Metformin may have an important role in diminishing the adverse consequences of obesity and metabolic impairments in patients with schizophrenia.
Trial registration: ClinicalTrials.gov NCT00816907.
Figures
Comment in
-
Metformin for antipsychotic-related weight gain and metabolic abnormalities: when, for whom, and for how long?Am J Psychiatry. 2013 Sep;170(9):947-52. doi: 10.1176/appi.ajp.2013.13060771. Am J Psychiatry. 2013. PMID: 24030606 No abstract available.
-
Metformin and Alzheimer's disease risk.Am J Psychiatry. 2014 Jan;171(1):119. doi: 10.1176/appi.ajp.2013.13091193. Am J Psychiatry. 2014. PMID: 24399432 No abstract available.
-
Response to Rosenfeld.Am J Psychiatry. 2014 Jan;171(1):119-20. doi: 10.1176/appi.ajp.2013.13091193r. Am J Psychiatry. 2014. PMID: 24399433 No abstract available.
-
Metformin for weight loss in schizophrenia: safe but not a panacea.Evid Based Ment Health. 2014 May;17(2):51-2. doi: 10.1136/eb-2014-101760. Epub 2014 Apr 1. Evid Based Ment Health. 2014. PMID: 24692251 No abstract available.
References
-
- Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, Haukka J. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) Lancet. 2009;374:620–627. - PubMed
-
- Osby U, Correia N, Brandt L, Ekbom A, Sparén P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. 2000;45:21–28. - PubMed
-
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. - PubMed
-
- Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, López-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rössler A, Grobbee DE. EUFEST study group: Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371:1085–1097. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical